Does Simultaneous Administration of Bivalent (Types 1 and 3) Oral Poliovirus Vaccine and Inactivated Poliovirus Vaccine Induce Mucosal Cross-immunity to Poliovirus Type 2?
- PMID: 30376088
- PMCID: PMC6206124
- DOI: 10.1093/cid/ciy604
Does Simultaneous Administration of Bivalent (Types 1 and 3) Oral Poliovirus Vaccine and Inactivated Poliovirus Vaccine Induce Mucosal Cross-immunity to Poliovirus Type 2?
Abstract
Background: Inactivated poliovirus vaccine (IPV) alone does not induce mucosal immunity. However, it was hypothesized that administration of IPV together with bivalent (types 1+3) oral poliovirus vaccine (bOPV) may stimulate mucosal cross-immunity to poliovirus type 2 (PV2).
Methods: Cuban infants were randomized to receive either one dose of IPV (Arm A); one dose of IPV with bOPV (Arm B) at about 6 months of age or no vaccine (Arm C). Subjects were challenged with one dose of trivalent OPV (tOPV); they were about 7 months old in arms A and B, and about 3 months old in arm C at a time of the tOPV challenge. Sera were collected before vaccination and 30 days after tOPV challenge and tested for presence of poliovirus neutralizing antibodies; stool samples were collected at days 0, 7, 14, 21 and 49 post-challenge and tested for presence of poliovirus.
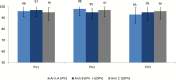
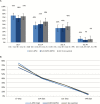
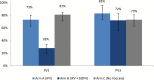
Results: We enrolled 333 children. Excretion of PV2 following tOPV challenge was highest on day 7 (75 [CI 95% = 65-82%], 68 [CI 95% = 58-75%] and 73 [CI 95% = 63-80%] for study arms A, B, and C respectively); excretion decreased with every subsequent stool sampling; no significant differences either in proportion of PV2 excretion or in its duration were observed between study arms.
Conclusions: There was no reduction in excretion of PV2 after tOPV challenge in children who had received IPV with bOPV when compared to those who had received IPV alone or no vaccine. Polio eradication program cannot assume any PV2 mucosal response with the current polio immunization schedule.
Clinical trials registration: The trial was registered with the Australian New Zealand Clinical Trials Registry and allocated trial number ACTRN12616000169448.
Figures
Similar articles
-
Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: an open-label randomised controlled trial.Lancet. 2016 Jul 9;388(10040):158-69. doi: 10.1016/S0140-6736(16)00703-0. Epub 2016 May 19. Lancet. 2016. PMID: 27212429 Clinical Trial.
-
Evaluation of vaccine derived poliovirus type 2 outbreak response options: A randomized controlled trial, Karachi, Pakistan.Vaccine. 2018 Mar 20;36(13):1766-1771. doi: 10.1016/j.vaccine.2018.02.051. Epub 2018 Feb 21. Vaccine. 2018. PMID: 29477307 Free PMC article.
-
Vaccine-induced mucosal immunity to poliovirus: analysis of cohorts from an open-label, randomised controlled trial in Latin American infants.Lancet Infect Dis. 2016 Dec;16(12):1377-1384. doi: 10.1016/S1473-3099(16)30169-4. Epub 2016 Sep 13. Lancet Infect Dis. 2016. PMID: 27638357 Free PMC article. Clinical Trial.
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. Cochrane Database Syst Rev. 2019. PMID: 31801180 Free PMC article.
-
Immunogenicity of New Primary Immunization Schedules With Inactivated Poliovirus Vaccine and Bivalent Oral Polio Vaccine for the Polio Endgame: A Review.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S35-S41. doi: 10.1093/cid/ciy633. Clin Infect Dis. 2018. PMID: 30376081 Free PMC article. Review.
Cited by
-
Intestinal mucosal immune responses induced by novel oral poliovirus vaccine type 2 and Sabin monovalent oral poliovirus vaccine type 2: an analysis of data from four clinical trials.Lancet Microbe. 2025 Jun;6(6):101028. doi: 10.1016/j.lanmic.2024.101028. Epub 2025 Apr 30. Lancet Microbe. 2025. PMID: 40318674 Free PMC article.
-
Persistence of immunity following a single dose of inactivated poliovirus vaccine: a phase 4, open label, non-randomised clinical trial.Lancet Microbe. 2023 Nov;4(11):e923-e930. doi: 10.1016/S2666-5247(23)00215-X. Epub 2023 Sep 26. Lancet Microbe. 2023. PMID: 37774729 Free PMC article. Clinical Trial.
-
Mucosal immunity to poliovirus.Mucosal Immunol. 2022 Jan;15(1):1-9. doi: 10.1038/s41385-021-00428-0. Epub 2021 Jul 8. Mucosal Immunol. 2022. PMID: 34239028 Free PMC article. Review.
-
Reduced mucosal immunity to poliovirus after cessation of trivalent oral polio vaccine.Hum Vaccin Immunother. 2021 Aug 3;17(8):2560-2567. doi: 10.1080/21645515.2021.1911213. Epub 2021 Apr 13. Hum Vaccin Immunother. 2021. PMID: 33848232 Free PMC article.
-
Co-Administration of Injected and Oral Vaccine Candidates Elicits Improved Immune Responses over Either Route Alone.Vaccines (Basel). 2020 Jan 21;8(1):37. doi: 10.3390/vaccines8010037. Vaccines (Basel). 2020. PMID: 31973150 Free PMC article.
References
-
- Cases of wild poliovirus by country and year Geneva: World Health Organization, 2017. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpoli...6 November 2017.
-
- Centers for Disease Control andPrevention. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mortal Wkly Rep 2001; 50:222–4. - PubMed
-
- Cases of wild poliovirus by country and year Geneva: World Health Organization, 2017. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpoli.... Accessed 9 November 2013.
-
- Más Lago P, Gary HE Jr, Pérez LS, et al. . Poliovirus detection in wastewater and stools following an immunization campaign in Havana, Cuba. Int J Epidemiol 2003; 32:772–7. - PubMed
-
- Huang QS, Greening G, Baker MG, et al. . Persistence of oral polio vaccine virus after its removal from the immunisation schedule in New Zealand. Lancet 2005; 366:394–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

