SMAD4 Is Essential for Human Cardiac Mesodermal Precursor Cell Formation
- PMID: 30376214
- PMCID: PMC7379516
- DOI: 10.1002/stem.2943
SMAD4 Is Essential for Human Cardiac Mesodermal Precursor Cell Formation
Abstract
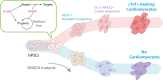
Understanding stage-specific molecular mechanisms of human cardiomyocyte (CM) progenitor formation and subsequent differentiation are critical to identify pathways that might lead to congenital cardiovascular defects and malformations. In particular, gene mutations in the transforming growth factor (TGF)β superfamily signaling pathways can cause human congenital heart defects, and murine loss of function studies of a central component in this pathway, Smad4, leads to early embryonic lethality. To define the role of SMAD4 at the earliest stages of human cardiogenesis, we generated SMAD4 mutant human embryonic stem cells (hESCs). Herein, we show that the loss of SMAD4 has no effect on hESC self-renewal, or neuroectoderm formation, but is essential for the formation of cardiac mesoderm, with a subsequent complete loss of CM formation during human ES cell cardiogenesis. Via transcriptional profiling, we show that SMAD4 mutant cell lines fail to generate cardiac mesodermal precursors, clarifying a role of NODAL/SMAD4 signaling in cardiac mesodermal precursor formation via enhancing the expression of primitive streak genes. Since SMAD4 relative pathways have been linked to congenital malformations, it will become of interest to determine whether these may due, in part, to defective cell fate decision during cardiac mesodermal precursor formation. Stem Cells 2018 Stem Cells 2019;37:216-225.
Keywords: Heart; Human embryonic stem cells; Mesoderm; SMAD4.
© 2018 The Authors. Stem Cells published by Wiley Periodicals, Inc. on behalf of AlphaMed Press 2018.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures
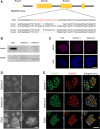
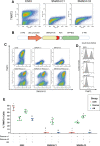
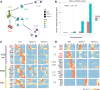
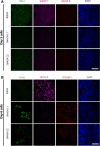
References
-
- Xu RH, Peck RM, Li DS et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods 2005;2:185–190. - PubMed
-
- James D, Levine AJ, Besser D et al. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 2005;132:1273–1282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

