Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer
- PMID: 30376427
- PMCID: PMC6553803
- DOI: 10.1200/JCO.18.00283
Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer
Erratum in
-
Errata.J Clin Oncol. 2019 Apr 10;37(11):942. doi: 10.1200/JCO.19.00517. J Clin Oncol. 2019. PMID: 30951645 Free PMC article. No abstract available.
Abstract
Purpose: Microsatellite instability (MSI) and/or mismatch repair deficiency (MMR-D) testing has traditionally been performed in patients with colorectal (CRC) and endometrial cancer (EC) to screen for Lynch syndrome (LS)-associated cancer predisposition. The recent success of immunotherapy in high-frequency MSI (MSI-H) and/or MMR-D tumors now supports testing for MSI in all advanced solid tumors. The extent to which LS accounts for MSI-H across heterogeneous tumor types is unknown. Here, we establish the prevalence of LS across solid tumors according to MSI status.
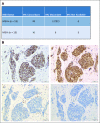
Methods: MSI status was determined using targeted next-generation sequencing, with tumors classified as MSI-H, MSI-indeterminate, or microsatellite-stable. Matched germline DNA was analyzed for mutations in LS-associated mismatch repair genes ( MLH1, MSH2, MSH6, PMS2, EPCAM). In patients with LS with MSI-H/I tumors, immunohistochemical staining for MMR-D was assessed.
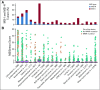
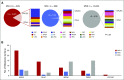
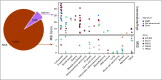
Results: Among 15,045 unique patients (more than 50 cancer types), LS was identified in 16.3% (53 of 326), 1.9% (13 of 699), and 0.3% (37 of 14,020) of patients with MSI-H, MSI-indeterminate, and microsatellite-stable tumors, respectively ( P < .001). Among patients with LS with MSI-H/I tumors, 50% (33 of 66) had tumors other than CRC/EC, including urothelial, prostate, pancreas, adrenocortical, small bowel, sarcoma, mesothelioma, melanoma, gastric, and germ cell tumors. In these patients with non-CRC/EC tumors, 45% (15 of 33) did not meet LS genetic testing criteria on the basis of personal/family history. Immunohistochemical staining of LS-positive MSI-H/I tumors demonstrated MMR-D in 98.2% (56 of 57) of available cases.
Conclusion: MSI-H/MMR-D is predictive of LS across a much broader tumor spectrum than currently appreciated. Given implications for cancer surveillance and prevention measures in affected families, these data support germline genetic assessment for LS for patients with an MSI-H/MMR-D tumor, regardless of cancer type or family cancer history.
Figures
Comment in
-
Tumor Testing for Microsatellite Instability to Identify Lynch Syndrome: New Insights Into an Old Diagnostic Strategy.J Clin Oncol. 2019 Feb 1;37(4):263-265. doi: 10.1200/JCO.18.01664. Epub 2018 Dec 14. J Clin Oncol. 2019. PMID: 30550362 No abstract available.
-
Lynch Syndrome: Widening the Net.Gastroenterology. 2019 Nov;157(5):1432-1434. doi: 10.1053/j.gastro.2019.09.028. Epub 2019 Oct 3. Gastroenterology. 2019. PMID: 31586568 No abstract available.
-
High predictability for identifying Lynch syndrome via microsatellite instability testing or immunohistochemistry in all Lynch-associated tumor types.Transl Cancer Res. 2019 Dec;8(Suppl 6):S559-S563. doi: 10.21037/tcr.2019.08.10. Transl Cancer Res. 2019. PMID: 32266124 Free PMC article. No abstract available.
References
-
- Hampel H, Frankel WL, Martin E, et al. : Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 352:1851-1860, 2005 - PubMed
-
- Hampel H, Frankel W, Panescu J, et al. : Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 66:7810-7817, 2006 - PubMed
-
- Gupta S, Provenzale D, Regenbogen SE, et al. : NCCN guidelines insights: Genetic/familial high-risk assessment: Colorectal, version 3.2017. J Natl Compr Canc Netw 15:1465-1475, 2017 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

