The temporal organization of mouse ultrasonic vocalizations
- PMID: 30376572
- PMCID: PMC6207298
- DOI: 10.1371/journal.pone.0199929
The temporal organization of mouse ultrasonic vocalizations
Abstract
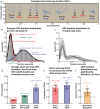
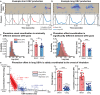
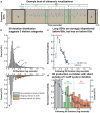
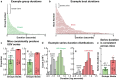
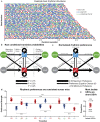
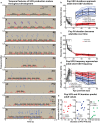
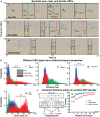
House mice, like many tetrapods, produce multielement calls consisting of individual vocalizations repeated in rhythmic series. In this study, we examine the multielement ultrasonic vocalizations (USVs) of adult male C57Bl/6J mice and specifically assess their temporal properties and organization. We found that male mice produce two classes of USVs which display unique temporal features and arise from discrete respiratory patterns. We also observed that nearly all USVs were produced in repetitive series exhibiting a hierarchical organization and a stereotyped rhythmic structure. Furthermore, series rhythmicity alone was determined to be sufficient for the mathematical discrimination of USVs produced by adult males, adult females, and pups, underscoring the known importance of call timing in USV perception. Finally, the gross spectrotemporal features of male USVs were found to develop continuously from birth and stabilize by P50, suggesting that USV production in infants and adults relies on common biological mechanisms. In conclusion, we demonstrate that the temporal organization of multielement mouse USVs is both stable and informative, and we propose that call timing be explicitly assessed when examining mouse USV production. Furthermore, this is the first report of putative USV classes arising from distinct articulatory patterns in mice, and is the first to empirically define multielement USV series and provide a detailed description of their temporal structure and development. This study therefore represents an important point of reference for the analysis of mouse USVs, a commonly used metric of social behavior in mouse models of human disease, and furthers the understanding of vocalization production in an accessible mammalian species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
CBA/CaJ mouse ultrasonic vocalizations depend on prior social experience.PLoS One. 2018 Jun 6;13(6):e0197774. doi: 10.1371/journal.pone.0197774. eCollection 2018. PLoS One. 2018. PMID: 29874248 Free PMC article.
-
Rates of female mouse ultrasonic vocalizations are low and are not modulated by estrous state during interactions with muted males.Sci Rep. 2025 Feb 26;15(1):6841. doi: 10.1038/s41598-025-91479-x. Sci Rep. 2025. PMID: 40000725 Free PMC article.
-
Social isolation produces no effect on ultrasonic vocalization production in adult female CBA/CaJ mice.PLoS One. 2019 Mar 5;14(3):e0213068. doi: 10.1371/journal.pone.0213068. eCollection 2019. PLoS One. 2019. PMID: 30835741 Free PMC article.
-
Determinants of the mouse ultrasonic vocal structure and repertoire.Neurosci Biobehav Rev. 2016 Jun;65:313-25. doi: 10.1016/j.neubiorev.2016.03.029. Epub 2016 Apr 7. Neurosci Biobehav Rev. 2016. PMID: 27060755 Review.
-
Translating mouse vocalizations: prosody and frequency modulation.Genes Brain Behav. 2011 Feb;10(1):4-16. doi: 10.1111/j.1601-183X.2010.00603.x. Genes Brain Behav. 2011. PMID: 20497235 Free PMC article. Review.
Cited by
-
5-MeO-DMT modifies innate behaviors and promotes structural neural plasticity in mice.Neuropsychopharmacology. 2023 Aug;48(9):1257-1266. doi: 10.1038/s41386-023-01572-w. Epub 2023 Apr 4. Neuropsychopharmacology. 2023. PMID: 37015972 Free PMC article.
-
Unsupervised discovery of family specific vocal usage in the Mongolian gerbil.Elife. 2024 Dec 16;12:RP89892. doi: 10.7554/eLife.89892. Elife. 2024. PMID: 39680425 Free PMC article.
-
The breath shape controls intonation of mouse vocalizations.Elife. 2024 Jul 4;13:RP93079. doi: 10.7554/eLife.93079. Elife. 2024. PMID: 38963785 Free PMC article.
-
Recurrent laryngeal nerve transection in mice results in translational upper airway dysfunction.J Comp Neurol. 2020 Mar 1;528(4):574-596. doi: 10.1002/cne.24774. Epub 2019 Oct 18. J Comp Neurol. 2020. PMID: 31512255 Free PMC article.
-
Maternal immune activation alters the sequential structure of ultrasonic communications in male rats.Brain Behav Immun Health. 2021 Jul 28;16:100304. doi: 10.1016/j.bbih.2021.100304. eCollection 2021 Oct. Brain Behav Immun Health. 2021. PMID: 34589796 Free PMC article.
References
-
- Noirot E, Pye D. Sound analysis of ultrasonic distress calls of mouse pups as a function of their age. Animal Behaviour. 1969;17:340–349. 10.1016/0003-3472(69)90020-7 - DOI
-
- Portfors CV. Types and Functions of Ultrasonic Vocalizations in Laboratory Rats and Mice. Journal of the American Association for Laboratory Animal Science. 2007;46(1):7. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

