Contribution of individual COPD assessment test (CAT) items to CAT total score and effects of pulmonary rehabilitation on CAT scores
- PMID: 30376861
- PMCID: PMC6208036
- DOI: 10.1186/s12955-018-1034-4
Contribution of individual COPD assessment test (CAT) items to CAT total score and effects of pulmonary rehabilitation on CAT scores
Abstract
Background: The COPD Assessment Test (CAT) contains eight items (cough, phlegm, chest tightness, breathlessness, limited activities, confidence leaving home, sleeplessness and energy). The current study aimed 1) to better understand the impact of the respiratory and non-respiratory CAT item scores on the CAT total score; and 2) to determine the impact of pulmonary rehabilitation (PR) on CAT items and CAT total score.
Methods: CAT total score of ≥10 or ≥ 18 points was used to classify patients as highly symptomatic, a decrease of 2 points was considered as clinically relevant improvement. 'Cough', 'phlegm', 'chest tightness', 'breathlessness' were defined as respiratory items; ≥3 points on each item was defined as highly symptomatic.
Results: In total, 497 clinically stable patients (55% male, age 64.0 (57.5-71.0) years, FEV1 46.0 (32.0-63.0)% predicted, CAT total score 22.0 (17.5-26.0) points) were included. 95% had CAT score ≥ 10 points and 75% ≥18 points. Respectively, 45% and 54% of subjects scored high on 3 or 4 of the respiratory CAT items. Following PR, 220 patients (57.7%) reported an improved health status as assessed by CAT total score (- 3.0 (- 7.0-1.0) points). Change in CAT item scores ranged from 0.0 (- 1.0-0.0) to - 1.0 (- 2.0-0.0) points) with best improvements in 'energy' (- 1.0 (- 2.0-0.0)points).
Conclusions: A substantial number of patients classified as highly symptomatic did not report a high level of respiratory symptoms, indicating that non-respiratory symptoms impact on disease classification and treatment algorithm. The impact of PR on CAT item scores varied by individual item.
Trial registration: Netherlands National Trial Register ( NTR3416 ). Registered 2 May 2012.
Keywords: COPD assessment test; GOLD classification; Health status; Pulmonary rehabilitation.
Conflict of interest statement
Ethics approval and consent to participate
The COPD, health status and co-morbidities (Chance) study was approved by the local ethics committee of Maastricht University Medical Centre+, The Netherlands (MEC11–3-070). All patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
FMEF discloses consulting and speaker fees from Novartis, GlaxoSmithKline, AstraZeneca, Chiesi, Boehringer Ingelheim and Teva. LEGWV discloses speaker fees from Chiesi, Novartis, GlaxoSmithKline and AstraZeneca. EFMW discloses consulting and speaker fees from Nycomed, Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Novartis and Chiesi. MAS discloses speaker fees from GlaxoSmithKline, Boehringer Ingelheim and AstraZeneca. SH-W and DJAJ have nothing to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
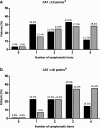
 ) and four non-respiratory items (limited activity, confidence leaving home, sleeplessness, energy;
) and four non-respiratory items (limited activity, confidence leaving home, sleeplessness, energy; ) on the CAT total score a) ≥10 points (n = 469) and b) ≥18 points (n = 373). X-as demonstrates the number of symptomatic (defined as ≥3 points) CAT items. A GOLD 2018 report [1]; B Smid et al. JAMDA 2017 [4]
) on the CAT total score a) ≥10 points (n = 469) and b) ≥18 points (n = 373). X-as demonstrates the number of symptomatic (defined as ≥3 points) CAT items. A GOLD 2018 report [1]; B Smid et al. JAMDA 2017 [4]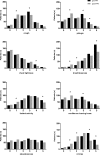
References
-
- Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. https://goldcopd.org/; 2018.
-
- Smid DE, Franssen FME, Gonik M, Miravitlles M, Casanova C, Cosio BG, de Lucas-Ramos P, Marin JM, Martinez C, Mir I, et al. Redefining Cut-Points for High Symptom Burden of the Global Initiative for Chronic Obstructive Lung Disease Classification in 18,577 Patients With Chronic Obstructive Pulmonary Disease. J Am Med Dir Assoc. 2017;18:1097 e1011–1097 e1024. doi: 10.1016/j.jamda.2016.08.002. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

