Endoplasmic reticulum resident oxidase ERO1-Lalpha promotes hepatocellular carcinoma metastasis and angiogenesis through the S1PR1/STAT3/VEGF-A pathway
- PMID: 30377291
- PMCID: PMC6207574
- DOI: 10.1038/s41419-018-1134-4
Endoplasmic reticulum resident oxidase ERO1-Lalpha promotes hepatocellular carcinoma metastasis and angiogenesis through the S1PR1/STAT3/VEGF-A pathway
Abstract
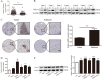
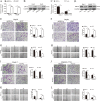
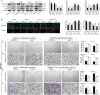
Mounting evidence demonstrates that expression of ERO1α, an endoplasmic reticulum (ER)-resident oxidase, is a poor prognosis factor in a variety of human cancers. However, the clinical relevance of ERO1α and its molecular mechanisms underlying tumor progression have not been determined for hepatocellular carcinoma (HCC). ERO1α expression levels in HCC tissues and cells were detected by quantitative real-time PCR and western blotting. ERO1α shRNAs and overexpression vector were transfected into HCC cells to downregulate or upregulate ERO1α expression. In vitro and in vivo assays were performed to investigate the function of ERO1α in invasion, metastasis, and angiogenesis of HCC. We found high ERO1α expression in HCC tissues and cells that was significantly associated with metastasis and poor clinicopathologic features of vascular invasion, advanced Edmondson Grade, and TNM stage. Loss-of-function and gain-of-function studies showed that ERO1α prompted migration, invasion, epithelial-mesenchymal transition (EMT), and angiogenesis of HCC cells both in vitro and in vivo. Further studies verified a positive correlation between ERO1α and S1PR1, upregulated in metastatic HCC tissues compared with HCC tissues without metastasis. S1PR1 knockdown markedly diminished the effects of ERO1α on HCC cell migration, invasion and vascular endothelial growth factor (VEGF) expression. Most importantly, ERO1α knockdown significantly repressed the death of HCC xenograft mouse models by reducing tumor distant metastasis, and host angiogenesis by suppressing the expression of S1PR1, p-STAT3, and VEGF-A in HCC cells. Our findings suggest that ERO1α is significantly correlated with reduced survival and poor prognosis, and promotes HCC metastasis and angiogenesis by triggering the S1PR1/STAT3/VEGF-A signaling pathway. ERO1α might be a novel candidate in HCC prognosis and therapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

