Human mast cells promote colon cancer growth via bidirectional crosstalk: studies in 2D and 3D coculture models
- PMID: 30377568
- PMCID: PMC6205014
- DOI: 10.1080/2162402X.2018.1504729
Human mast cells promote colon cancer growth via bidirectional crosstalk: studies in 2D and 3D coculture models
Abstract
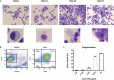
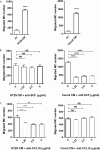
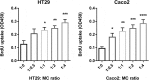
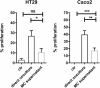
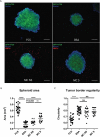
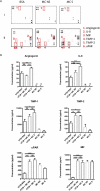
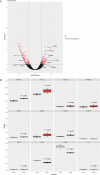
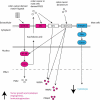
Chronic inflammation drives the development of colorectal cancer (CRC), where tumor-infiltrating immune cells interact with cancer cells in a dynamic crosstalk. Mast cells (MC), one of earliest recruited immune cells, accumulate in CRC tissues and their density is correlated with cancer progression. However, the exact contribution of MC in CRC and their interaction with colon cancer cells is poorly understood. Here, we investigated the impact of primary human MC and their mediators on colon cancer growth using 2D and 3D coculture models. Primary human MC were generated from peripheral CD34+ stem cells. Transwell chambers were used to analyze MC chemotaxis to colon cancer. Colon cancer cells HT29 and Caco2 differentially recruited MC by releasing CCL15 or SCF, respectively. Using BrdU proliferation assays, we demonstrated that MC can directly support colon cancer proliferation and this effect was mediated by their cellular crosstalk. 3D coculture models with cancer spheroids further confirmed the pro-tumor effect of MC on colon cancer growth, where direct cell-cell contact is dispensable and increased production of multiple soluble mediators was detected. Moreover, TLR2 stimulation of MC promoted stronger growth of colon cancer spheroids. By examining the transcriptome profile of colon cancer-cocultured MC versus control MC, we identified several MC marker genes, which were deregulated in expression. Our study provides an advanced in vitro model to investigate the role of human MC in cancer. Our data support the detrimental role of MC in CRC development and provide a molecular insight into the cellular crosstalk between MC and colon cancer cells.
Keywords: 3D coculture; CCL15; Human mast cells; TLR2; cellular crosstalk; colorectal cancer; gene expression; stem cell factor.
Figures
Similar articles
-
A Transcriptomic Insight into the Impact of Colon Cancer Cells on Mast Cells.Int J Mol Sci. 2019 Apr 4;20(7):1689. doi: 10.3390/ijms20071689. Int J Mol Sci. 2019. PMID: 30987352 Free PMC article.
-
Mast cell-T cell axis alters development of colitis-dependent and colitis-independent colorectal tumours: potential for therapeutically targeting via mast cell inhibition.J Immunother Cancer. 2022 Oct;10(10):e004653. doi: 10.1136/jitc-2022-004653. J Immunother Cancer. 2022. PMID: 36220303 Free PMC article.
-
Human intestinal fibroblasts prevent apoptosis in human intestinal mast cells by a mechanism independent of stem cell factor, IL-3, IL-4, and nerve growth factor.J Immunol. 2004 Jan 1;172(1):260-7. doi: 10.4049/jimmunol.172.1.260. J Immunol. 2004. PMID: 14688333
-
Mast cells interact directly with colorectal cancer cells to promote epithelial-to-mesenchymal transition.bioRxiv [Preprint]. 2025 Mar 19:2025.03.19.644113. doi: 10.1101/2025.03.19.644113. bioRxiv. 2025. PMID: 40166179 Free PMC article. Preprint.
-
The significant role of mast cells in cancer.Cancer Metastasis Rev. 2011 Mar;30(1):45-60. doi: 10.1007/s10555-011-9286-z. Cancer Metastasis Rev. 2011. PMID: 21287360 Review.
Cited by
-
Mast cells in colorectal cancer tumour progression, angiogenesis, and lymphangiogenesis.Front Immunol. 2023 Jul 11;14:1209056. doi: 10.3389/fimmu.2023.1209056. eCollection 2023. Front Immunol. 2023. PMID: 37497234 Free PMC article. Review.
-
Multiregion single-cell sequencing reveals the transcriptional landscape of the immune microenvironment of colorectal cancer.Clin Transl Med. 2021 Jan;11(1):e253. doi: 10.1002/ctm2.253. Clin Transl Med. 2021. PMID: 33463049 Free PMC article.
-
Immune-Related Gene Expression and Cytokine Secretion Is Reduced Among African American Colon Cancer Patients.Front Oncol. 2020 Sep 2;10:1498. doi: 10.3389/fonc.2020.01498. eCollection 2020. Front Oncol. 2020. PMID: 32983990 Free PMC article.
-
An In Vitro Model of Mast Cell Recruitment and Activation by Breast Cancer Cells Supports Anti-Tumoral Responses.Int J Mol Sci. 2020 Jul 26;21(15):5293. doi: 10.3390/ijms21155293. Int J Mol Sci. 2020. PMID: 32722549 Free PMC article.
-
Levels of circulating mast cell progenitors and tumour‑infiltrating mast cells in patients with colorectal cancer.Oncol Rep. 2022 May;47(5):89. doi: 10.3892/or.2022.8300. Epub 2022 Mar 16. Oncol Rep. 2022. PMID: 35293596 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
