Cytotoxic activity of the genus Ferula (Apiaceae) and its bioactive constituents
- PMID: 30377589
- PMCID: PMC6204145
Cytotoxic activity of the genus Ferula (Apiaceae) and its bioactive constituents
Abstract
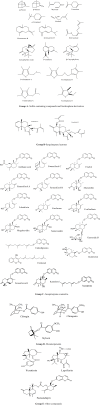
Objective: The genus Ferula L. includes perennial flowering plants belonging to the Apiaceae family. This genus is a rich source of biologically active phytochemicals such as sulfur-containing derivatives, coumarins, sesquiterpenes, sesquiterpene lactones, sesquiterpene coumarins, glucuronic acid, galactose, arabinose, rhamnose, and daucane esters. Over the last decade, considerable attention has been paid to biological activities of these compounds; it is assumed that the most prominent biological features of the genus Ferula are their cytotoxic effects. This article discusses cytotoxic activity of the genus Ferula and their important compounds.
Materials and methods: In this mini-review article, papers published from 1990 to April 2016 were included and the following information was discussed; cytotoxic activity of the genus Ferula and their important compounds, the type of cell line used in vitro, concentrations of the extracts/active compound that were used, and the underlying mechanisms of action through which Ferula-related chemicals induced cytotoxicity. In addition, we explained different mechanisms of action through which the active constituents isolated from Ferula, could decrease cellular growth.
Conclusion: It is highly recommended that potent and effective compounds that were isolated from Ferula plants and found to be appropriate as adjuvant therapy for certain diseases, should be identified. Also, the versatile biological activities of sesquiterpene coumarins suggest them as promising agents with a broad range of biological applications to be used in the future.
Keywords: Apiaceae; Biological activity; Cytotoxicity; Farnesiferol C; Ferula; Sesquiterpene coumarin; Umbelliprenin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Metabolic Profile, Bioactivities, and Variations in the Chemical Constituents of Essential Oils of the Ferula Genus (Apiaceae).Front Pharmacol. 2021 Mar 12;11:608649. doi: 10.3389/fphar.2020.608649. eCollection 2020. Front Pharmacol. 2021. PMID: 33776754 Free PMC article. Review.
-
Recent Advances on Bioactive Constituents in Ferula.Drug Dev Res. 2017 Nov;78(7):321-331. doi: 10.1002/ddr.21402. Epub 2017 Aug 8. Drug Dev Res. 2017. PMID: 28786182 Review.
-
Biologically active sesquiterpene coumarins from Ferula species.Phytother Res. 2011 Mar;25(3):315-23. doi: 10.1002/ptr.3311. Epub 2010 Oct 28. Phytother Res. 2011. PMID: 21031633 Review.
-
Biological activities of farnesiferol C: a review.J Asian Nat Prod Res. 2018 Jan;20(1):27-35. doi: 10.1080/10286020.2017.1379997. Epub 2017 Sep 26. J Asian Nat Prod Res. 2018. PMID: 28948835 Review.
-
Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects.Molecules. 2020 Dec 7;25(23):5768. doi: 10.3390/molecules25235768. Molecules. 2020. PMID: 33297504 Free PMC article. Review.
Cited by
-
Chemical Composition Analysis, Cytotoxic, Antimicrobial and Antioxidant Activities of Physalis angulata L.: A Comparative Study of Leaves and Fruit.Molecules. 2022 Feb 22;27(5):1480. doi: 10.3390/molecules27051480. Molecules. 2022. PMID: 35268579 Free PMC article.
-
Assessment of the acute and subacute toxicity of the aqueous extract of Moroccan Ferula communis fruit in a mouse model.Saudi Pharm J. 2023 Aug;31(8):101701. doi: 10.1016/j.jsps.2023.101701. Epub 2023 Jul 7. Saudi Pharm J. 2023. PMID: 37576855 Free PMC article.
-
A Review of Anticancer Potential of Conferone, Diversin and Ferutinin; Which One is Stronger for Cancer Therapy?Anticancer Agents Med Chem. 2025;25(6):378-387. doi: 10.2174/0118715206328175241022081832. Anticancer Agents Med Chem. 2025. PMID: 39482921 Review.
-
Antiausterity Activity of Secondary Metabolites from the Roots of Ferula hezarlalehzarica against the PANC-1 Human Pancreatic Cancer Cell Line.J Nat Prod. 2020 Apr 24;83(4):1099-1106. doi: 10.1021/acs.jnatprod.9b01109. Epub 2020 Mar 12. J Nat Prod. 2020. PMID: 32163286 Free PMC article.
-
Anticancer potential of Ferula assa-foetida and its constituents, a powerful plant for cancer therapy.World J Biol Chem. 2023 Mar 27;14(2):28-39. doi: 10.4331/wjbc.v14.i2.28. World J Biol Chem. 2023. PMID: 37034135 Free PMC article. Review.
References
-
- Aas Z, Babaei E, Feizi MAH, Dehghan G. Anti-proliferative and apoptotic effects of dendrosomal farnesiferol c on gastric cancer cells. Asian Pac J Cancer Prev. 2015;16:5325–5329. - PubMed
-
- Aldaghi L, Rad A, Arab A, Kasaian J, Iranshahi M, Sadr A, Soltani F. In silico and in vitro evaluation of cytotoxic activities of farnesiferol c and microlobin on MCF-7, HeLa and KYSE Cell Lines. Drug Res. 2016;66:532–538. - PubMed
-
- Altmann K-H, Gertsch J. Anticancer drugs from nature—natural products as a unique source of new microtubule-stabilizing agents. Nat Prod Rep. 2007;24:327–357. - PubMed
-
- Appendino G, Maxia L, Bascope M, Houghton PJ, Sanchez-Duffhues G, Muñoz E, Sterner O. A meroterpenoid NF-κB inhibitor and drimane sesquiterpenoids from asafetida. J Nat Prod. 2006;69:1101–1104. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous