Bone marrow-derived mesenchymal stem cells inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia after arterial injury in rats
- PMID: 30377672
- PMCID: PMC6202691
- DOI: 10.1016/j.bbrep.2018.10.001
Bone marrow-derived mesenchymal stem cells inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia after arterial injury in rats
Abstract
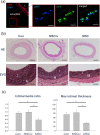
We investigated whether mesenchymal stem cell (MSC)-based treatment could inhibit neointimal hyperplasia in a rat model of carotid arterial injury and explored potential mechanisms underlying the positive effects of MSC therapy on vascular remodeling/repair. Sprague-Dawley rats underwent balloon injury to their right carotid arteries. After 2 days, we administered cultured MSCs from bone marrow of GFP-transgenic rats (0.8 × 106 cells, n = 10) or vehicle (controls, n = 10) to adventitial sites of the injured arteries. As an additional control, some rats received a higher dose of MSCs by systemic infusion (3 × 106 cells, tail vein; n = 4). Local vascular MSC administration significantly prevented neointimal hyperplasia (intima/media ratio) and reduced the percentage of Ki67 + proliferating cells in arterial walls by 14 days after treatment, despite little evidence of long-term MSC engraftment. Notably, systemic MSC infusion did not alter neointimal formation. By immunohistochemistry, compared with neointimal cells of controls, cells in MSC-treated arteries expressed reduced levels of embryonic myosin heavy chain and RM-4, an inflammatory cell marker. In the presence of platelet-derived growth factor (PDGF-BB), conditioned medium from MSCs increased p27 protein levels and significantly attenuated VSMC proliferation in culture. Furthermore, MSC-conditioned medium suppressed the expression of inflammatory cytokines and RM-4 in PDGF-BB-treated VSMCs. Thus, perivascular administration of MSCs may improve restenosis after vascular injury through paracrine effects that modulate VSMC inflammatory phenotype.
Keywords: CdM, conditioned medium CCM: complete culture medium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ICAM-1, intercellular adhesion molecule-1; IEL, internal elastic lamina EEL: external elastic lamina; IL-6, interleukin-6 MCP-1: monocyte chemoattractant protein-1; MSC, mesenchymal stem cell GFP: green fluorescence protein; Mesenchymal stem cells; Neointimal hyperplasia; Stem cell-secreted factors; VSMC, vascular smooth muscle cell PDGF: platelet-derived growth factor; Vascular smooth muscle cells.
Figures



Similar articles
-
Nobiletin Inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration and attenuates neointimal hyperplasia in a rat carotid artery injury model.Drug Dev Res. 2014 Dec;75(8):489-96. doi: 10.1002/ddr.21230. Epub 2014 Nov 28. Drug Dev Res. 2014. PMID: 25452110
-
Exosomes derived from mesenchymal stem cells inhibit neointimal hyperplasia by activating the Erk1/2 signalling pathway in rats.Stem Cell Res Ther. 2020 Jun 8;11(1):220. doi: 10.1186/s13287-020-01676-w. Stem Cell Res Ther. 2020. PMID: 32513275 Free PMC article.
-
Exosomes from mesenchymal stem cells expressing miR-125b inhibit neointimal hyperplasia via myosin IE.J Cell Mol Med. 2019 Feb;23(2):1528-1540. doi: 10.1111/jcmm.14060. Epub 2018 Nov 28. J Cell Mol Med. 2019. PMID: 30484954 Free PMC article.
-
A review of the current state in neointimal hyperplasia development following endovascular intervention and minor emphasis on new horizons in immunotherapy.Transl Clin Pharmacol. 2023 Dec;31(4):191-201. doi: 10.12793/tcp.2023.31.e18. Epub 2023 Nov 22. Transl Clin Pharmacol. 2023. PMID: 38196998 Free PMC article. Review.
-
Epigenetic Regulation of Vascular Smooth Muscle Cell Phenotype Switching in Atherosclerotic Artery Remodeling: A Mini-Review.Front Genet. 2021 Aug 5;12:719456. doi: 10.3389/fgene.2021.719456. eCollection 2021. Front Genet. 2021. PMID: 34422021 Free PMC article. Review.
Cited by
-
The functional mechanism of bone marrow-derived mesenchymal stem cells in the treatment of animal models with Alzheimer's disease: crosstalk between autophagy and apoptosis.Stem Cell Res Ther. 2022 Mar 3;13(1):90. doi: 10.1186/s13287-022-02765-8. Stem Cell Res Ther. 2022. PMID: 35241159 Free PMC article. Review.
-
Effects of norepinephrine‑induced activation of rat vascular adventitial fibroblasts on proliferation and migration of BMSCs involved in vascular remodeling.Exp Ther Med. 2023 May 3;25(6):290. doi: 10.3892/etm.2023.11989. eCollection 2023 Jun. Exp Ther Med. 2023. PMID: 37206559 Free PMC article.
-
The effect of endothelial progenitor cell transplantation on neointimal hyperplasia and reendothelialisation after balloon catheter injury in rat carotid arteries.Stem Cell Res Ther. 2021 Feb 3;12(1):99. doi: 10.1186/s13287-021-02135-w. Stem Cell Res Ther. 2021. PMID: 33536065 Free PMC article.
-
Bioengineering Strategies for Treating Neointimal Hyperplasia in Peripheral Vasculature: Innovations and Challenges.Adv Healthc Mater. 2025 Mar;14(7):e2401056. doi: 10.1002/adhm.202401056. Epub 2025 Jan 31. Adv Healthc Mater. 2025. PMID: 39888207 Free PMC article. Review.
-
Efficient differentiation of vascular smooth muscle cells from Wharton's Jelly mesenchymal stromal cells using human platelet lysate: A potential cell source for small blood vessel engineering.World J Stem Cells. 2020 Mar 26;12(3):203-221. doi: 10.4252/wjsc.v12.i3.203. World J Stem Cells. 2020. PMID: 32266052 Free PMC article.
References
-
- Serruys P.W., Kutryk M.J., Ong A.T. Coronary-artery stents. N. Engl. J. Med. 2006;354:483–495. - PubMed
-
- Finn A.V., Nakazawa G., Joner M. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler. Thromb. Vasc. Biol. 2007;27:1500–1510. - PubMed
-
- Phinney D.G., Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

