Topical Glycopyrronium Tosylate for the Treatment of Primary Axillary Hyperhidrosis: Patient-Reported Outcomes from the ATMOS-1 and ATMOS-2 Phase III Randomized Controlled Trials
- PMID: 30378087
- PMCID: PMC6516143
- DOI: 10.1007/s40257-018-0395-0
Topical Glycopyrronium Tosylate for the Treatment of Primary Axillary Hyperhidrosis: Patient-Reported Outcomes from the ATMOS-1 and ATMOS-2 Phase III Randomized Controlled Trials
Abstract
Background: Glycopyrronium tosylate (GT) is a topical anticholinergic approved in the USA for primary axillary hyperhidrosis in patients aged ≥ 9 years. GT was evaluated for primary axillary hyperhidrosis in replicate, randomized, double-blind, vehicle-controlled, phase III trials. GT reduced sweating severity and production versus vehicle and was generally well tolerated.
Objective: Our objective was to evaluate patient-reported outcomes (PROs) from these trials.
Methods: Patients aged ≥ 9 years with primary axillary hyperhidrosis ≥ 6 months, gravimetrically measured sweat production ≥ 50 mg/5 min in each axilla, Axillary Sweating Daily Diary (ASDD) Item 2 severity score ≥ 4, and Hyperhidrosis Disease Severity Scale (HDSS) score ≥ 3 were randomized 2:1 to GT 3.75% or vehicle applied once daily to each axilla for 4 weeks. The 4-item ASDD, 6 Weekly Impact (WI) items, Patient Global Impression of Change (PGIC), HDSS, and Dermatology Life Quality Index (DLQI) were utilized.
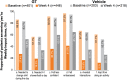
Results: In the pooled population, 463 patients were randomized to GT and 234 to vehicle; 426 (92.0%) and 225 (96.2%) completed the trials. At baseline, most patients considered their axillary sweating to be at least moderate in severity, impact, and bothersomeness (ASDD items 2, 3, and 4, respectively). Improvement was substantially greater for GT than for vehicle at every study week, and, at week 4, ASDD scores improved from baseline by 62.6 versus 34.0% (severity), 65.5 versus 40.3% (impact), and 65.4 versus 39.0% (bothersomeness). Improvements favoring GT versus vehicle also occurred for WI items, PGIC, HDSS, and DLQI.
Conclusions: PRO results demonstrated that GT reduced the disease burden of primary axillary hyperhidrosis.
Trial registration: Clinicaltrials.gov; ATMOS-1 (NCT02530281), ATMOS-2 (NCT02530294).
Conflict of interest statement
Ethical Approval/Informed Consent
ATMOS-1 was conducted in the USA and Germany; ATMOS-2 was conducted in the USA. Trial protocols and informed consent forms were approved by local institutional review boards or independent ethics committees on 13 May 2015, and the first patients were enrolled in July 2015 (ATMOS-1) and August 2015 (ATMOS-2). The trials were registered on ClinicalTrials.gov on 21 August 2015 (ATMOS-1 [NCT02530281] and ATMOS-2 [NCT02530294]). Both trials were carried out in accordance with Good Clinical Practice and the Declaration of Helsinki.
Conflicts of Interest
DMP has received honoraria for consultancy from Brickell Biotech, Inc., Biofrontera AG, Celgene, Dermira, Inc., DUSA Pharmaceuticals, Inc., LEO Pharma, Novartis, Promius Pharma, LLC, Regeneron Pharmaceuticals, Inc., Sanofi, TheraVida, Inc., and Valeant Pharmaceuticals International, Inc; honoraria for participating on an advisory board for Pfizer, Inc.; investigator grants/research funding from Abbott Laboratories; Amgen, Inc.; Brickell Biotech, Inc.; Celgene; Dermavant Sciences; Eli Lilly and Company; LEO Pharma; Merck & Co, Inc.; Novartis; Novo Nordisk A/S; Ortho Dermatologics; Peplin, Inc.; Photocure ASA; Promius Pharma, LLC; Regeneron Pharmaceuticals, Inc.; Stiefel Laboratories; and Valeant Pharmaceuticals International, Inc.; and investigator honoraria from LEO Pharma and Pfizer, Inc. AAH has received investigator research funding from Dermira, Inc. (paid to the UTHealth McGovern Medical School, Houston); advisory board honoraria from Dermira, Inc.; research funding (all monies paid to the UTHealth McGovern Medical School) from Allergan, Plc., Amgen, Inc., Cassiopea, Celgene, Dermavant Sciences, Eli Lilly and Company, Galderma S.A., GSK, Plc., LEO Pharma, Mayne Pharma, Medimetriks Pharmaceuticals, Novan, Inc., Promius Pharma, LLC, and Vanda Pharmaceuticals; honoraria from Amgen, GSK, Plc., Pfizer, Inc., and Valeant Pharmaceutics International, Inc. JD and RG are employees of Dermira, Inc. JQ is a consultant for Dermira, Inc. and an employee of QST Consultations. DAG is a consultant and investigator for Dermira, Inc. and an investigator for Allergan, ATACAMA, Brickell, Galderma, Revance, and Sienna.
Figures



Similar articles
-
Glycopyrronium tosylate in pediatric primary axillary hyperhidrosis: Post hoc analysis of efficacy and safety findings by age from two phase three randomized controlled trials.Pediatr Dermatol. 2019 Jan;36(1):89-99. doi: 10.1111/pde.13723. Epub 2018 Nov 19. Pediatr Dermatol. 2019. PMID: 30451318 Free PMC article. Clinical Trial.
-
Topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: Results from the ATMOS-1 and ATMOS-2 phase 3 randomized controlled trials.J Am Acad Dermatol. 2019 Jan;80(1):128-138.e2. doi: 10.1016/j.jaad.2018.07.002. Epub 2018 Jul 10. J Am Acad Dermatol. 2019. PMID: 30003988 Clinical Trial.
-
A 44-Week Open-Label Study Evaluating Safety and Efficacy of Topical Glycopyrronium Tosylate in Patients with Primary Axillary Hyperhidrosis.Am J Clin Dermatol. 2019 Aug;20(4):593-604. doi: 10.1007/s40257-019-00446-6. Am J Clin Dermatol. 2019. PMID: 31111409 Free PMC article. Clinical Trial.
-
Efficacy and safety of topical glycopyrronium bromide in treating axillary hyperhidrosis: systematic review and meta-analysis.Sci Rep. 2024 Oct 19;14(1):24537. doi: 10.1038/s41598-024-74430-4. Sci Rep. 2024. PMID: 39424822 Free PMC article.
-
Glycopyrronium Tosylate (Qbrexza) for Hyperhidrosis.Skin Therapy Lett. 2019 Mar;24(2):1-3. Skin Therapy Lett. 2019. PMID: 30970203 Review.
Cited by
-
Bibliometric Analysis in Hyperhidrosis: Recent Publication Trends from 2015 to 2025.Skin Appendage Disord. 2025 May 8:1-16. doi: 10.1159/000545767. Online ahead of print. Skin Appendage Disord. 2025. PMID: 40510941 Free PMC article.
-
Glycopyrronium tosylate in pediatric primary axillary hyperhidrosis: Post hoc analysis of efficacy and safety findings by age from two phase three randomized controlled trials.Pediatr Dermatol. 2019 Jan;36(1):89-99. doi: 10.1111/pde.13723. Epub 2018 Nov 19. Pediatr Dermatol. 2019. PMID: 30451318 Free PMC article. Clinical Trial.
-
Long-term efficacy and safety of topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: Post hoc pediatric subgroup analysis from a 44-week open-label extension study.Pediatr Dermatol. 2020 May;37(3):490-497. doi: 10.1111/pde.14135. Epub 2020 Mar 8. Pediatr Dermatol. 2020. PMID: 32147881 Free PMC article. Clinical Trial.
-
Management Strategies Of Palmar Hyperhidrosis: Challenges And Solutions.Clin Cosmet Investig Dermatol. 2019 Oct 4;12:733-744. doi: 10.2147/CCID.S210973. eCollection 2019. Clin Cosmet Investig Dermatol. 2019. PMID: 31632121 Free PMC article.
-
Limited Systemic Exposure with Topical Glycopyrronium Tosylate in Primary Axillary Hyperhidrosis.Clin Pharmacokinet. 2021 May;60(5):665-676. doi: 10.1007/s40262-020-00975-y. Epub 2021 Jan 12. Clin Pharmacokinet. 2021. PMID: 33433785 Free PMC article. Clinical Trial.
References
-
- Amir M, Arish A, Weinstein Y, Pfeffer M, Levy Y. Impairment in quality of life among patients seeking surgery for hyperhidrosis (excessive sweating): preliminary results. Isr J Psychiatry Relat Sci. 2000;37:25–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

