Traumatic brain injury-induced neuronal damage in the somatosensory cortex causes formation of rod-shaped microglia that promote astrogliosis and persistent neuroinflammation
- PMID: 30378170
- PMCID: PMC7542609
- DOI: 10.1002/glia.23523
Traumatic brain injury-induced neuronal damage in the somatosensory cortex causes formation of rod-shaped microglia that promote astrogliosis and persistent neuroinflammation
Abstract
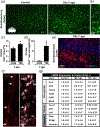
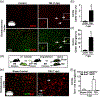
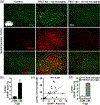
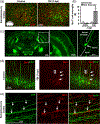
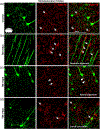
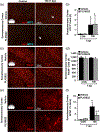
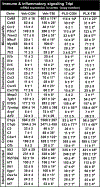
Microglia undergo dynamic structural and transcriptional changes during the immune response to traumatic brain injury (TBI). For example, TBI causes microglia to form rod-shaped trains in the cerebral cortex, but their contribution to inflammation and pathophysiology is unclear. The purpose of this study was to determine the origin and alignment of rod microglia and to determine the role of microglia in propagating persistent cortical inflammation. Here, diffuse TBI in mice was modeled by midline fluid percussion injury (FPI). Bone marrow chimerism and BrdU pulse-chase experiments revealed that rod microglia derived from resident microglia with limited proliferation. Novel data also show that TBI-induced rod microglia were proximal to axotomized neurons, spatially overlapped with dense astrogliosis, and aligned with apical pyramidal dendrites. Furthermore, rod microglia formed adjacent to hypertrophied microglia, which clustered among layer V pyramidal neurons. To better understand the contribution of microglia to cortical inflammation and injury, microglia were eliminated prior to TBI by CSF1R antagonism (PLX5622). Microglial elimination did not affect cortical neuron axotomy induced by TBI, but attenuated rod microglial formation and astrogliosis. Analysis of 262 immune genes revealed that TBI caused profound cortical inflammation acutely (8 hr) that progressed in nature and complexity by 7 dpi. For instance, gene expression related to complement, phagocytosis, toll-like receptor signaling, and interferon response were increased 7 dpi. Critically, these acute and chronic inflammatory responses were prevented by microglial elimination. Taken together, TBI-induced neuronal injury causes microglia to structurally associate with neurons, augment astrogliosis, and propagate diverse and persistent inflammatory/immune signaling pathways.
Keywords: CSF1R antagonist; astrocytes; fluid percussion injury; microglia; neuroinflammation; traumatic brain injury.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Similar articles
-
Traumatic Brain Injury Causes Chronic Cortical Inflammation and Neuronal Dysfunction Mediated by Microglia.J Neurosci. 2021 Feb 17;41(7):1597-1616. doi: 10.1523/JNEUROSCI.2469-20.2020. Epub 2021 Jan 15. J Neurosci. 2021. PMID: 33452227 Free PMC article.
-
Diffuse traumatic brain injury induced stimulator of interferons (STING) signaling in microglia drives cortical neuroinflammation, neuronal dysfunction, and impaired cognition.J Neuroinflammation. 2025 Apr 30;22(1):128. doi: 10.1186/s12974-025-03451-1. J Neuroinflammation. 2025. PMID: 40307881 Free PMC article.
-
Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury.J Neuroinflammation. 2017 Jul 24;14(1):143. doi: 10.1186/s12974-017-0917-3. J Neuroinflammation. 2017. PMID: 28738820 Free PMC article.
-
Microglia-mediated neuroinflammation in traumatic brain injury: a review.Mol Biol Rep. 2024 Oct 19;51(1):1073. doi: 10.1007/s11033-024-09995-4. Mol Biol Rep. 2024. PMID: 39425760 Review.
-
Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury.Brain Behav Immun. 2018 Jul;71:9-17. doi: 10.1016/j.bbi.2018.03.033. Epub 2018 Mar 27. Brain Behav Immun. 2018. PMID: 29601944 Review.
Cited by
-
The Bidirectional Relationship Between Sleep and Inflammation Links Traumatic Brain Injury and Alzheimer's Disease.Front Neurosci. 2020 Aug 25;14:894. doi: 10.3389/fnins.2020.00894. eCollection 2020. Front Neurosci. 2020. PMID: 32982677 Free PMC article. Review.
-
Revisiting Excitotoxicity in Traumatic Brain Injury: From Bench to Bedside.Pharmaceutics. 2022 Jan 8;14(1):152. doi: 10.3390/pharmaceutics14010152. Pharmaceutics. 2022. PMID: 35057048 Free PMC article. Review.
-
Deplete and repeat: microglial CSF1R inhibition and traumatic brain injury.Front Cell Neurosci. 2024 Feb 21;18:1352790. doi: 10.3389/fncel.2024.1352790. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38450286 Free PMC article. Review.
-
Infliximab Can Improve Traumatic Brain Injury by Suppressing the Tumor Necrosis Factor Alpha Pathway.Mol Neurobiol. 2021 Jun;58(6):2803-2811. doi: 10.1007/s12035-021-02293-1. Epub 2021 Jan 27. Mol Neurobiol. 2021. PMID: 33501626 Review.
-
The evolving pathophysiology of TBI and the advantages of temporally-guided combination therapies.Neurochem Int. 2024 Nov;180:105874. doi: 10.1016/j.neuint.2024.105874. Epub 2024 Oct 2. Neurochem Int. 2024. PMID: 39366429 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

