Tenocyte cell density, migration, and extracellular matrix deposition with amniotic suspension allograft
- PMID: 30378182
- PMCID: PMC6587843
- DOI: 10.1002/jor.24173
Tenocyte cell density, migration, and extracellular matrix deposition with amniotic suspension allograft
Abstract
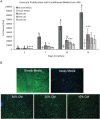
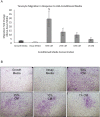
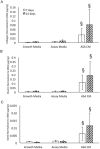
Amniotic suspension allografts (ASA), derived from placental tissues, contain particulated amniotic membrane and amniotic fluid cells. Recently, ASA and other placental-derived allografts have been used in orthopaedic applications, including tendinopathies and tendon injuries. The purpose of this study was to determine the potential effects of ASA on tenocyte cell density, migration, and responses to inflammatory stimuli. Tenocyte cell density was measured using AlamarBlue over multiple time points, while migration was determined using a Boyden chamber assay. Deposition of ECM markers were measured using BioColor kits. Gene expression and protein production of cytokines and growth factors following stimulus with pro-inflammatory IL-1β and TNF-α was measured using qPCR and ELISAs. Conditioned media (CM) was made from ASA and used for all assays in this study. In vitro, ASA CM treatment significantly promoted tenocyte increases in cell density and migration compared to assay media controls. ASA CM also increased the deposition of extracellular matrix (ECM) proteins, including collagen, elastin, and sGAG. Following inflammatory stimulation and treatment with ASA CM, tenocytes downregulated IL-8 gene expression, a pro-inflammatory cytokine normally elevated during the inflammatory phase of tendon healing. Additionally, tenocytes treated with ASA CM had significantly lower protein levels of TGF-β1 compared to controls. This study evaluated ASA and its effect on tenocytes; specifically, treatment with ASA resulted in increased cell density, more robust migration and matrix deposition, and some alteration of inflammatory targets. © 2018 The Authors. Journal of Orthopaedic Research® Published by Wiley Periodicals, Inc. on behalf of Orthopaedic Research Society. J Orthop Res 37:412-420, 2019.
Keywords: amniotic suspension allograft; regenerative; repair; tendon; tenocytes.
© 2018 The Authors. Journal of Orthopaedic Research® Published by Wiley Periodicals, Inc. on behalf of Orthopaedic Research Society.
Figures


Similar articles
-
Evaluation of two distinct placental-derived membranes and their effect on tenocyte responses in vitro.J Tissue Eng Regen Med. 2019 Aug;13(8):1316-1330. doi: 10.1002/term.2876. Epub 2019 Jun 13. J Tissue Eng Regen Med. 2019. PMID: 31062484 Free PMC article.
-
Amniotic Suspension Allograft Modulates Inflammation in a Rat Pain Model of Osteoarthritis.J Orthop Res. 2020 May;38(5):1141-1149. doi: 10.1002/jor.24559. Epub 2019 Dec 19. J Orthop Res. 2020. PMID: 31814175 Free PMC article.
-
Immunomodulatory effects of amniotic membrane matrix incorporated into collagen scaffolds.J Biomed Mater Res A. 2016 Jun;104(6):1332-42. doi: 10.1002/jbm.a.35663. Epub 2016 Feb 8. J Biomed Mater Res A. 2016. PMID: 26799369 Free PMC article.
-
Effect of micro-RNA on tenocytes and tendon-related gene expression: A systematic review.J Orthop Res. 2018 Nov;36(11):2823-2829. doi: 10.1002/jor.24064. Epub 2018 Jul 13. J Orthop Res. 2018. PMID: 29873411
-
The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: new insights.Scand J Med Sci Sports. 2011 Jun;21(3):337-51. doi: 10.1111/j.1600-0838.2010.01265.x. Epub 2011 Jan 7. Scand J Med Sci Sports. 2011. PMID: 21210861 Review.
Cited by
-
Species variations in tenocytes' response to inflammation require careful selection of animal models for tendon research.Sci Rep. 2021 Jun 14;11(1):12451. doi: 10.1038/s41598-021-91914-9. Sci Rep. 2021. PMID: 34127759 Free PMC article.
-
Synergistic Effects of Insulin-like Growth Factor-1 and Platelet-Derived Growth Factor-BB in Tendon Healing.Int J Mol Sci. 2025 Apr 24;26(9):4039. doi: 10.3390/ijms26094039. Int J Mol Sci. 2025. PMID: 40362278 Free PMC article.
-
A decellularized flowable placental connective tissue matrix supports cellular functions of human tenocytes in vitro.J Exp Orthop. 2022 Jul 18;9(1):69. doi: 10.1186/s40634-022-00509-4. J Exp Orthop. 2022. PMID: 35849201 Free PMC article.
-
Treatment with Human Amniotic Suspension Allograft Improves Tendon Healing in a Rat Model of Collagenase-Induced Tendinopathy.Cells. 2019 Nov 8;8(11):1411. doi: 10.3390/cells8111411. Cells. 2019. PMID: 31717431 Free PMC article.
-
Placental Tissues as Biomaterials in Regenerative Medicine.Biomed Res Int. 2022 Apr 21;2022:6751456. doi: 10.1155/2022/6751456. eCollection 2022. Biomed Res Int. 2022. PMID: 35496035 Free PMC article. Review.
References
-
- Sabella N. 1913. Use of fetal membranes in skin grafting. Med Rec 83:478–480.
-
- Riboh JC, Saltzman BM, Yanke AB, et al. 2016. Human amniotic membrane‐derived products in sports medicine: basic science, early results, and potential clinical applications. Am J Sports Med 44:2425–2434. - PubMed
-
- Bryant‐Greenwood GD. 1998. The extracellular matrix of the human fetal membranes: structure and function. Placenta 19:1–11. - PubMed
-
- Hao Y, Ma DH‐K, Hwang DG, et al. 2000. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 19:348–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

