An update on enterovirus 71 infection and interferon type I response
- PMID: 30378208
- PMCID: PMC7169063
- DOI: 10.1002/rmv.2016
An update on enterovirus 71 infection and interferon type I response
Abstract
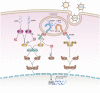
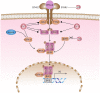
Enteroviruses are members of Pichornaviridae family consisting of human enterovirus group A, B, C, and D as well as nonhuman enteroviruses. Hand, foot, and mouth disease (HFMD) is a serious disease which is usually seen in the Asia-Pacific region in children. Enterovirus 71 and coxsackievirus A16 are two important viruses responsible for HFMD which are members of group A enterovirus. IFN α and β are two cytokines, which have a major activity in the innate immune system against viral infections. Most of the viruses have some weapons against these cytokines. EV71 has two main proteases called 2A and 3C, which are important for polyprotein processing and virus maturation. Several studies have indicated that they have a significant effect on different cellular pathways such as interferon production and signaling pathway. The aim of this study was to investigate the latest findings about the interaction of 2A and 3C protease of EV71 and IFN production/signaling pathway and their inhibitory effects on this pathway.
Keywords: 2A protease; 3C protease introduction; enterovirus 71; interferon type I.
© 2018 John Wiley & Sons, Ltd.
Conflict of interest statement
The authors have no competing interest.
Figures
Similar articles
-
Essential Role of Enterovirus 2A Protease in Counteracting Stress Granule Formation and the Induction of Type I Interferon.J Virol. 2019 May 1;93(10):e00222-19. doi: 10.1128/JVI.00222-19. Print 2019 May 15. J Virol. 2019. PMID: 30867299 Free PMC article.
-
Disruption of MDA5-Mediated Innate Immune Responses by the 3C Proteins of Coxsackievirus A16, Coxsackievirus A6, and Enterovirus D68.J Virol. 2017 Jun 9;91(13):e00546-17. doi: 10.1128/JVI.00546-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28424289 Free PMC article.
-
Activation of Host Cellular Signaling and Mechanism of Enterovirus 71 Viral Proteins Associated with Hand, Foot and Mouth Disease.Viruses. 2022 Oct 4;14(10):2190. doi: 10.3390/v14102190. Viruses. 2022. PMID: 36298746 Free PMC article. Review.
-
Enterovirus 71 and coxsackievirus A16 3C proteases: binding to rupintrivir and their substrates and anti-hand, foot, and mouth disease virus drug design.J Virol. 2011 Oct;85(19):10319-31. doi: 10.1128/JVI.00787-11. Epub 2011 Jul 27. J Virol. 2011. PMID: 21795339 Free PMC article.
-
[Research into the Pathogenicity of Enterovirus 71].Bing Du Xue Bao. 2015 Mar;31(2):192-6. Bing Du Xue Bao. 2015. PMID: 26164947 Review. Chinese.
Cited by
-
Modulation of plasmacytoid dendritic cell and CD4+ T cell differentiation accompanied by upregulation of the cholinergic anti-inflammatory pathway induced by enterovirus 71.Arch Virol. 2024 Mar 13;169(4):73. doi: 10.1007/s00705-024-05974-z. Arch Virol. 2024. PMID: 38472498
-
TBK1 and IRF3 are potential therapeutic targets in Enterovirus A71-associated diseases.PLoS Negl Trop Dis. 2023 Jan 10;17(1):e0011001. doi: 10.1371/journal.pntd.0011001. eCollection 2023 Jan. PLoS Negl Trop Dis. 2023. PMID: 36626364 Free PMC article.
-
Enterovirus A71 capsid protein VP1 increases blood-brain barrier permeability and virus receptor vimentin on the brain endothelial cells.J Neurovirol. 2020 Feb;26(1):84-94. doi: 10.1007/s13365-019-00800-8. Epub 2019 Sep 11. J Neurovirol. 2020. PMID: 31512144 Free PMC article.
-
The EV71 2A protease occupies the central cleft of SETD3 and disrupts SETD3-actin interaction.Nat Commun. 2024 May 16;15(1):4176. doi: 10.1038/s41467-024-48504-w. Nat Commun. 2024. PMID: 38755176 Free PMC article.
-
IFN-β1b induces OAS3 to inhibit EV71 via IFN-β1b/JAK/STAT1 pathway.Virol Sin. 2022 Oct;37(5):676-684. doi: 10.1016/j.virs.2022.07.013. Epub 2022 Aug 5. Virol Sin. 2022. PMID: 35934228 Free PMC article.
References
-
- Zoll J, Heus HA, van Kuppeveld FJ, Melchers WJ. The structure–function relationship of the enterovirus 3′‐UTR. Virus Res. 2009;139(2):209‐216. - PubMed
-
- Ho M, Chen E‐R, Hsu K‐H, et al. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999;341(13):929‐935. - PubMed
-
- Chen Y‐C, Yu C‐K, Wang Y‐F, Liu C‐C, Su I‐J, Lei H‐Y. A murine oral enterovirus 71 infection model with central nervous system involvement. J Gen Virol. 2004;85(1):69‐77. - PubMed
-
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26(1):91‐107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

