Varenicline and nabilone in tobacco and cannabis co-users: effects on tobacco abstinence, withdrawal and a laboratory model of cannabis relapse
- PMID: 30378231
- PMCID: PMC8167505
- DOI: 10.1111/adb.12664
Varenicline and nabilone in tobacco and cannabis co-users: effects on tobacco abstinence, withdrawal and a laboratory model of cannabis relapse
Abstract
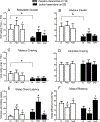
Tobacco and cannabis co-users (T+CUs) have poor cannabis cessation outcomes, but the mechanisms underlying this are not well understood. This laboratory study examined the effects of (1) the partial nicotinic agonist, varenicline, on tobacco cessation among T+CUs, and (2) varenicline, alone, and when combined with the cannabinoid agonist nabilone, on cannabis withdrawal and a laboratory model of cannabis relapse. Non-treatment-seeking T+CUs were randomized to active-varenicline or placebo-varenicline, and completed a 15-day outpatient phase; varenicline was titrated to 1 mg BID during days 1-8, and participants were instructed to abstain from tobacco during days 9-15. Participants then moved inpatient for 16 days, where they continued their outpatient medication and tobacco abstinence. Inpatient testing included two, 8-day medication periods, where active-nabilone and placebo-nabilone were administered in counterbalanced order, and measures of acute cannabis effects (days 1-2), withdrawal (days 4-5) and 'relapse' (days 6-8) were collected. Participants in the active-varenicline group were more likely to achieve cotinine-verified tobacco abstinence during the outpatient period versus placebo-varenicline group (46 percent versus 24 percent, respectively), and also reported less mood disturbance and cigarette craving while inpatient. Active-nabilone attenuated cannabis withdrawal in both groups but did not affect cannabis relapse. Regression analyses revealed that two tobacco-related variables, i.e. age of first cigarette use, and cigarette craving while inpatient, were independent predictors of cannabis relapse outcomes. Thus, varenicline holds promise in this population, as a tool to examine the effects of tobacco abstinence on cannabis use outcomes, and as a component of smoking cessation treatments targeting T+CUs.
Keywords: cannabis; pharmacotherapy; relapse; tobacco; varenicline; withdrawal.
© 2018 Society for the Study of Addiction.
Figures
Similar articles
-
Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users.Psychopharmacology (Berl). 2016 Jul;233(13):2469-78. doi: 10.1007/s00213-016-4298-6. Epub 2016 Apr 16. Psychopharmacology (Berl). 2016. PMID: 27085870 Free PMC article. Clinical Trial.
-
Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse.Neuropsychopharmacology. 2013 Jul;38(8):1557-65. doi: 10.1038/npp.2013.54. Epub 2013 Feb 26. Neuropsychopharmacology. 2013. PMID: 23443718 Free PMC article.
-
Cannabis and Alcohol Co-Use in a Smoking Cessation Pharmacotherapy Trial for Adolescents and Emerging Adults.Nicotine Tob Res. 2020 Jul 16;22(8):1374-1382. doi: 10.1093/ntr/ntz170. Nicotine Tob Res. 2020. PMID: 31612956 Free PMC article. Clinical Trial.
-
Current pharmacologic treatments for smoking cessation and new agents undergoing clinical trials.Ther Adv Respir Dis. 2019 Jan-Dec;13:1753466619875925. doi: 10.1177/1753466619875925. Ther Adv Respir Dis. 2019. PMID: 31533544 Free PMC article. Review.
-
[Smoking cessation: Pharmacological strategies different from standard treatments].Rev Pneumol Clin. 2018 Sep;74(4):205-214. doi: 10.1016/j.pneumo.2018.04.008. Rev Pneumol Clin. 2018. PMID: 29773262 Review. French.
Cited by
-
Clinical management of cannabis withdrawal.Addiction. 2022 Jul;117(7):2075-2095. doi: 10.1111/add.15743. Epub 2022 Jan 10. Addiction. 2022. PMID: 34791767 Free PMC article. Review.
-
Support Provided by Stop-Smoking Practitioners to Co-users of Tobacco and Cannabis: A Qualitative Study.Nicotine Tob Res. 2024 Jan 1;26(1):23-30. doi: 10.1093/ntr/ntad115. Nicotine Tob Res. 2024. PMID: 37429576 Free PMC article.
-
Evidence for the Endocannabinoid System as a Therapeutic Target in the Treatment of Cannabis Use Disorder.Curr Addict Rep. 2020 Dec;7:545-552. doi: 10.1007/s40429-020-00342-8. Epub 2020 Nov 9. Curr Addict Rep. 2020. PMID: 33816054 Free PMC article.
-
Responses to social evaluative stress in regular cannabis smokers.J Psychopharmacol. 2021 Jul;35(7):833-840. doi: 10.1177/0269881120972337. Epub 2021 Feb 7. J Psychopharmacol. 2021. PMID: 33554736 Free PMC article.
-
[Cannabis use and cannabis use disorders].Nervenarzt. 2024 Sep;95(9):781-796. doi: 10.1007/s00115-024-01722-5. Epub 2024 Aug 12. Nervenarzt. 2024. PMID: 39134752 Review. German.
References
-
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D et al. (2016). Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. The Lancet 387: 2507–2520. - PubMed
-
- Badiani A, Boden JM., De Pirro S, Fergusson DM, Horwood LJ, Harold GT (2015). Tobacco smoking and cannabis use in a longitudinal birth cohort: evidence of reciprocal causal relationships. Drug Alcohol Depend 150, 69–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

