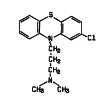
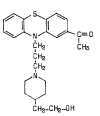
Chlorpromazine versus piperacetazine for schizophrenia
- PMID: 30378678
- PMCID: PMC6517193
- DOI: 10.1002/14651858.CD011709.pub2
Chlorpromazine versus piperacetazine for schizophrenia
Abstract
Background: Schizophrenia is a severe mental disorder with a prevalence of about 1% among the general population. It is listed among the top 10 causes of disability-adjusted life years (DALYs) worldwide. Antipsychotics are the mainstay treatment. Piperacetazine has been reported to be as clinically effective as chlorpromazine, a well established 'benchmark' antipsychotic, for people with schizophrenia. However, the side effect profiles of these antipsychotics differ and it is important that an evidence base is available comparing the benefits, and potential harms of these two antipsychotics.
Objectives: To assess the clinical and side effects of chlorpromazine for people with schizophrenia and schizophrenia-like psychoses in comparison with piperacetazine.
Search methods: We searched the Cochrane Schizophrenia Group's Trials Register (6 June 2015 and 8 October 2018) which is based on regular searches of CINAHL, CENTRAL, BIOSIS, AMED, Embase, PubMed, MEDLINE, PsycINFO and registries of clinical trials. There are no language, date, document type, or publication status limitations for inclusion of records in the register.
Selection criteria: We included randomised controlled trials (RCTs) focusing on chlorpromazine versus piperacetazine for people with schizophrenia, reporting useable data.
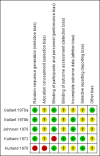
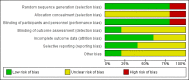
Data collection and analysis: We extracted data independently. For binary outcomes, we calculated risk ratio (RR) and its 95% confidence interval (CI), on an intention-to-treat basis. For continuous data, we estimated the mean difference (MD) between groups and its 95% CI. We employed a fixed-effect model for analyses. We assessed risk of bias for included studies and created 'Summary of findings' tables using GRADE.
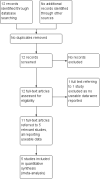
Main results: We found 12 records referring to six trials. We included five trials, all from the 1970s, randomising 343 participants. We excluded one trial. The overall methodology and data reporting by the trials was poor. Only short-term data were available.Results from the included trials found that, in terms of global state improvement, when rated by a psychiatrist, there was no clear difference between chlorpromazine and piperacetazine (RR 0.90, 95% CI 0.80 to 1.02; participants = 208; studies = 2; very low-quality evidence). One trial reported change scores on the mental state scale Brief Psychiatric Rating Scale (BPRS); no clear difference was observed (MD -0.40, 95% CI -1.41 to 0.61; participants = 182; studies = 1; very low-quality evidence). Chlorpromazine appears no worse or better than piperacetazine regarding adverse effects. In both treatment groups, around 60% of participants experienced some sort of adverse effect (RR 1.00, 95% CI 0.75 to 1.33; participants = 74; studies = 3; very low-quality evidence), with approximately 40% of these participants experiencing some parkinsonism-type movement disorder (RR 0.95, CI 0.61 to 1.49; participants = 106; studies = 3; very low-quality evidence). No clear difference in numbers of participants leaving the study early for any reason was observed (RR 0.50, 95% CI 0.10 to 2.56; participants = 256; studies = 4; very low-quality evidence). No trial reported data for change in negative symptoms or economic costs.
Authors' conclusions: The results of this review show chlorpromazine and piperacetazine may have similar clinical efficacy, but data are based on very small numbers of participants and the evidence is very low quality. We can not make firm conclusions based on such data. Currently, should clinicians and people with schizophrenia need to choose between chlorpromazine and piperacetazine they should be aware there is no good quality evidence to base decisions. More high quality research is needed.
Conflict of interest statement
Mahin Eslami Shahrbabaki: none known Rahim Sharafkhani: none known Reza Dehnavieh: none known Leila Vali: none known
Figures
Update of
- doi: 10.1002/14651858.CD011709
Similar articles
-
Electroconvulsive therapy for treatment-resistant schizophrenia.Cochrane Database Syst Rev. 2019 Mar 19;3(3):CD011847. doi: 10.1002/14651858.CD011847.pub2. Cochrane Database Syst Rev. 2019. PMID: 30888709 Free PMC article.
-
Modafinil for people with schizophrenia or related disorders.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD008661. doi: 10.1002/14651858.CD008661.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828767 Free PMC article.
-
Acetylsalicylic acid (aspirin) for schizophrenia.Cochrane Database Syst Rev. 2019 Aug 10;8(8):CD012116. doi: 10.1002/14651858.CD012116.pub2. Cochrane Database Syst Rev. 2019. PMID: 31425623 Free PMC article.
-
Cognitive behavioural therapy plus standard care versus standard care plus other psychosocial treatments for people with schizophrenia.Cochrane Database Syst Rev. 2018 Nov 15;11(11):CD008712. doi: 10.1002/14651858.CD008712.pub3. Cochrane Database Syst Rev. 2018. PMID: 30480760 Free PMC article.
-
Cognitive behavioural therapy plus standard care versus standard care for people with schizophrenia.Cochrane Database Syst Rev. 2018 Dec 20;12(12):CD007964. doi: 10.1002/14651858.CD007964.pub2. Cochrane Database Syst Rev. 2018. PMID: 30572373 Free PMC article.
Cited by
-
Chlorpromazine versus clotiapine for schizophrenia.Cochrane Database Syst Rev. 2017 Apr 7;4(4):CD011810. doi: 10.1002/14651858.CD011810.pub2. Cochrane Database Syst Rev. 2017. PMID: 28387925 Free PMC article.
-
Chlorpromazine versus penfluridol for schizophrenia.Cochrane Database Syst Rev. 2017 Sep 23;9(9):CD011831. doi: 10.1002/14651858.CD011831.pub2. Cochrane Database Syst Rev. 2017. PMID: 28940256 Free PMC article.
-
PELP1 Suppression Inhibits Gastric Cancer Through Downregulation of c-Src-PI3K-ERK Pathway.Front Oncol. 2020 Feb 13;9:1423. doi: 10.3389/fonc.2019.01423. eCollection 2019. Front Oncol. 2020. PMID: 32117782 Free PMC article.
-
Drug Repurposing Screen for Compounds Inhibiting the Cytopathic Effect of SARS-CoV-2.Front Pharmacol. 2021 Jan 25;11:592737. doi: 10.3389/fphar.2020.592737. eCollection 2020. Front Pharmacol. 2021. PMID: 33708112 Free PMC article.
-
Chlorpromazine versus metiapine for schizophrenia.Cochrane Database Syst Rev. 2017 Mar 25;3(3):CD011655. doi: 10.1002/14651858.CD011655.pub2. Cochrane Database Syst Rev. 2017. PMID: 28349512 Free PMC article.
References
References to studies included in this review
Gallant 1970a {published data only}
-
- Gallant DM, Bishop MP. Piperacetazine (quide): a controlled evaluation of the elixir in chronic schizophrenic patients. Current Therapeutic Research, Clinical and Experimental 1970;12(6):387‐9. - PubMed
-
- Gallant DM, Bishop MP. Piperacetazine versus chlorpromazine (liquid). Psychopharmacology Bulletin 1970;7(1):38‐40.
Gallant 1970b {published data only}
-
- Gallant DM, Bishop MP. Piperacetazine versus chlorpromazine (tablet). Psychopharmacology Bulletin 1970;6(4):101‐3.
Johnson 1970 {published data only}
-
- Johnson AC. Piperacetazine (liquid) versus chlorpromazine. Psychopharmacology Bulletin 1970;7(1):55‐7.
Kulkarni 1972 {published data only}
-
- Johnson AC, Kulkarni AS. Piperacetazine and chlorpromazine: a comparison. American Journal of Psychiatry 1973;130(5):603‐5. - PubMed
-
- Kiev A, Guclu B, Kulkarni AS. Evaluation of piperacetazine (quide) injection in acute schizophrenics. Current Therapeutic Research, Clinical and Experimental 1972;14(7):376‐80. - PubMed
-
- Kulkarni AS. Clinical effectiveness of piperacetazine injection in schizophrenic patients: a controlled study. Advances in Biochemical Psychopharmacology 1974;9:691‐9. - PubMed
-
- McLaughlin B, Kulkarni AS. Comparative evaluation of injectable chlorpromazine and piperacetazine. Psychosomatics 1973;14:220‐1. - PubMed
-
- Simeon J, Wadud A, Itil T. A comparison of phenothiazines in managing aggressive episodes in schizophrenic patients. Hospital and Community Psychiatry 1975;26(9):574. - PubMed
Kurland 1970 {published data only}
-
- Kurland O. Piperacetazine versus Chlorpromazine‐revision of summary reported on 2/13/70 with additional subjects. Psychopharmacology Bulletin 1970;7(1):57‐60.
-
- Kurland O. Piperacetazine versus chlorpromazine. Psychopharmacology Bulletin 1970;6(4):107‐9.
References to studies excluded from this review
Small 1970 {published data only}
-
- Small S. Piperacetazine (liquid) versus chlorpromazine. Psychopharmacology Bulletin 1970;7(1):52‐4.
Additional references
Adams 2005
Adams 2014
Ahmed 2010
Almerie 2007
Altman 1996
Anonymous 1971
-
- Anonymous. Evaluation of a new antipsychotic agent. Piperacetazine (quide). JAMA 1971;215(5):783‐4. - PubMed
Barber 2002
-
- Barber CC, Neese DT, Coyne L, Fultz J, Fonagy P. The target symptom rating: A brief clinical measure of acute psychiatric symptoms in children and adolescents. Journal of Clinical Child and Adolescent Psychology 2002 May 1;31(2):181‐92. - PubMed
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] - PubMed
Busner 2007
Carpenter 1994
-
- Carpenter WTJ, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330(10):681‐90. [PUBMED: 8107719] - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Eight International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne D, Altman DG, Higgins JP, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. - PubMed
Goldstein 1976
-
- Goldstein SE, Birnbom F. Piperacetazine versus thioridazine in the treatment of organic brain disease: a controlled double‐blind study. Journal of the American Geriatrics Society 1976;24(8):355‐8. - PubMed
Gulliford 1999
-
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hutton 2009
-
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986.
Kishimoto 2013
Kusumi 2015
-
- Kusumi I, Boku S, Takahashi Y. Psychopharmacology of atypical antipsychotic drugs: From the receptor binding profile to neuroprotection and neurogenesis. Psychiatry and Clinical Neurosciences 2015;69(5):243‐58. [PUBMED: 25296946] - PubMed
Leon 2006
-
- Leon AC, Mallinckrodt CH, Chuang‐Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001‐5. [PUBMED: 16905632] - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] - PubMed
Leucht 2005b
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Leucht 2008
Liu 2009
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812.
Palmer 2005
-
- Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia: a re‐examination. Archives of General Psychiatry 2005;62:247‐53. - PubMed
Rada 1972
-
- Rada RT, Donlon PT. Piperacetazine vs. thioridazine for the control of schizophrenia in outpatients. Psychosomatics 1972;13(6):373‐6. [PUBMED: 4153002] - PubMed
Rossler 2005
-
- Rossler W, Salize HJ, Os J, Riecher‐Rossler A. Size of burden of schizophrenia and psychotic disorders. European neuropsychopharmacology 2005;15(4):399‐409. [PUBMED: 15925493] - PubMed
Saha 2007
-
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time?. Archives of General Psychiatry 2007;64:1123‐31. - PubMed
Saha 2013
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shokraneh 2017
Shokraneh 2018
-
- Shokraneh F, Adams CE. Gallstone, snake venom and witchcraft for schizophrenia: the challenges of classifying [schizophrenia] trials. Evidence‐Based Medicine 2018;23(Suppl. 1):A18. [DOI: 10.1136/bmjebm-2018-111024.36] - DOI
Sterne 2011
-
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
Xia 2009
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

