Cell-specific Retention and Action of Pheophorbide-based Photosensitizers in Human Lung Cancer Cells
- PMID: 30378688
- PMCID: PMC6496943
- DOI: 10.1111/php.13043
Cell-specific Retention and Action of Pheophorbide-based Photosensitizers in Human Lung Cancer Cells
Abstract
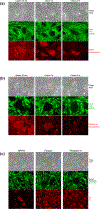
This study determined in primary cultures of human lung cancer cells the cell specificity of chlorin-based photosensitizers. Epithelial cells (ECs) preferentially retained 3-[1-hexyloxyethyl]-2-devinylpyropheophorbide-a (HPPH) and related structural variants. Tumor-associated fibroblasts (Fb) differ from EC by a higher efflux rate of HPPH. Immunoblot analyses indicated dimerization of STAT3 as a reliable biomarker of the photoreaction. Compared to mitochondria/ER-localized photoreaction by HPPH, the photoreaction by lysosomally targeted HPPH-lactose showed a trend toward lower STAT3 cross-linking. Lethal consequence of the photoreaction differed between EC and Fb with the latter cells being more resistant. A survey of lung tumor cases indicated a large quantitative range by which EC retains HPPH. The specificity of HPPH retention defined in vitro could be confirmed in vivo in selected cases grown as xenografts. HPPH retention as a function of the tetrapyrrole structure was evaluated by altering side groups on the porphyrin macrocycle. The presence or absence of a carboxylic acid at position 172 proved to be critical. A benzyl group at position 20 enhanced retention in a subset of cancer cells with low HPPH binding. This study indicated experimental tools that are potentially effective in defining the photosensitizer preference and application for individual patient's cancer lesions.
© 2018 The American Society of Photobiology.
Figures

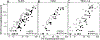






Similar articles
-
Structural and Epimeric Isomers of HPPH [3-Devinyl 3-{1-(1-hexyloxy) ethyl}pyropheophorbide-a]: Effects on Uptake and Photodynamic Therapy of Cancer.ACS Chem Biol. 2017 Apr 21;12(4):933-946. doi: 10.1021/acschembio.7b00023. Epub 2017 Feb 15. ACS Chem Biol. 2017. PMID: 28165706
-
Tumor cell-specific retention of photosensitizers determines the outcome of photodynamic therapy for head and neck cancer.J Photochem Photobiol B. 2022 Sep;234:112513. doi: 10.1016/j.jphotobiol.2022.112513. Epub 2022 Jul 8. J Photochem Photobiol B. 2022. PMID: 35841739
-
Conjugation of 2-(1'-hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH) to carbohydrates changes its subcellular distribution and enhances photodynamic activity in vivo.J Med Chem. 2009 Jul 23;52(14):4306-18. doi: 10.1021/jm9001617. J Med Chem. 2009. PMID: 19507863 Free PMC article.
-
Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications.Int J Mol Sci. 2023 Dec 12;24(24):17404. doi: 10.3390/ijms242417404. Int J Mol Sci. 2023. PMID: 38139233 Free PMC article. Review.
-
Mesoscopic fluorescence tomography of a photosensitizer (HPPH) 3D biodistribution in skin cancer.Acad Radiol. 2014 Feb;21(2):271-80. doi: 10.1016/j.acra.2013.11.009. Acad Radiol. 2014. PMID: 24439340 Review.
Cited by
-
Chiral Alkyl Groups at Position 3(1') of Pyropheophorbide-a Specify Uptake and Retention by Tumor Cells and Are Essential for Effective Photodynamic Therapy.J Med Chem. 2021 Apr 22;64(8):4787-4809. doi: 10.1021/acs.jmedchem.0c02090. Epub 2021 Apr 6. J Med Chem. 2021. PMID: 33822622 Free PMC article.
-
Impact of mono- and di-β-galactose moieties in in vitro / in vivo anticancer efficacy of pyropheophorbide-carbohydrate conjugates by photodynamic therapy.Eur J Med Chem Rep. 2022 Aug;5:100047. doi: 10.1016/j.ejmcr.2022.100047. Epub 2022 Apr 18. Eur J Med Chem Rep. 2022. PMID: 36568335 Free PMC article.
-
Tumor Cell-Specific Retention and Photodynamic Action of Erlotinib-Pyropheophorbide Conjugates.Int J Mol Sci. 2022 Sep 21;23(19):11081. doi: 10.3390/ijms231911081. Int J Mol Sci. 2022. PMID: 36232384 Free PMC article.
-
Charged groups on pyropheophorbide-based photosensitizers dictate uptake by tumor cells and photodynamic therapy efficacy.J Photochem Photobiol B. 2022 Feb;227:112375. doi: 10.1016/j.jphotobiol.2021.112375. Epub 2021 Dec 15. J Photochem Photobiol B. 2022. PMID: 34968800 Free PMC article.
-
Excitation of a Single Compound by Light and Ultrasound Enhanced the Long-Term Cure of Mice Bearing Prostate Tumors.Int J Mol Sci. 2023 Jun 25;24(13):10624. doi: 10.3390/ijms241310624. Int J Mol Sci. 2023. PMID: 37445799 Free PMC article.
References
-
- Loewen GM, Pandey R, Bellnier D, Henderson B, Dougherty T. Endobronchial photodynamic therapy for lung cancer. Lasers Surg Med. 2006;38(5):364–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

