Step-Wise Chondrogenesis of Human Induced Pluripotent Stem Cells and Purification Via a Reporter Allele Generated by CRISPR-Cas9 Genome Editing
- PMID: 30378731
- PMCID: PMC6312762
- DOI: 10.1002/stem.2931
Step-Wise Chondrogenesis of Human Induced Pluripotent Stem Cells and Purification Via a Reporter Allele Generated by CRISPR-Cas9 Genome Editing
Abstract
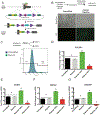
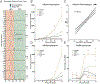
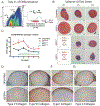
The differentiation of human induced pluripotent stem cells (hiPSCs) to prescribed cell fates enables the engineering of patient-specific tissue types, such as hyaline cartilage, for applications in regenerative medicine, disease modeling, and drug screening. In many cases, however, these differentiation approaches are poorly controlled and generate heterogeneous cell populations. Here, we demonstrate cartilaginous matrix production in three unique hiPSC lines using a robust and reproducible differentiation protocol. To purify chondroprogenitors (CPs) produced by this protocol, we engineered a COL2A1-GFP knock-in reporter hiPSC line by CRISPR-Cas9 genome editing. Purified CPs demonstrated an improved chondrogenic capacity compared with unselected populations. The ability to enrich for CPs and generate homogenous matrix without contaminating cell types will be essential for regenerative and disease modeling applications. Stem Cells 2019;37:65-76.
Keywords: CRISPR-Cas9; Chondrocyte; Chondrogenesis; Differentiation; Genome editing; iPSC.
© AlphaMed Press 2018.
Conflict of interest statement
Disclosure
V.W. and F. G. discloses employment/leadership position with Cytex Therapeutics Inc. All other authors declared not conflict of interest.
Figures



References
-
- van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis and Cartilage. 2012;20:223–32. - PubMed
-
- Tonge DP, Pearson MJ, Jones SW. The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics Osteoarthritis and Cartilage. Elsevier Ltd; 2014;22:1–13. - PubMed
-
- Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat. Rev. Dis. Primers 2016;2:16072. - PubMed
-
- Katz J Total joint replacement in osteoarthritis. Best Pract Res Cl Rh. 2006;20:145–53. - PubMed
-
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med 1994;331:889–95. - PubMed
Publication types
MeSH terms
Grants and funding
- R43 AR072166/AR/NIAMS NIH HHS/United States
- R21 DA041878/DA/NIDA NIH HHS/United States
- R01 AG046927/AG/NIA NIH HHS/United States
- R01 AR048182/AR/NIAMS NIH HHS/United States
- R01 AG015768/AG/NIA NIH HHS/United States
- U01 HG007900/HG/NHGRI NIH HHS/United States
- R21 AR067467/AR/NIAMS NIH HHS/United States
- R41 GM119914/GM/NIGMS NIH HHS/United States
- R03 AR061042/AR/NIAMS NIH HHS/United States
- R21 AR065956/AR/NIAMS NIH HHS/United States
- DP2 OD008586/OD/NIH HHS/United States
- R21 AR072870/AR/NIAMS NIH HHS/United States
- R01 DA036865/DA/NIDA NIH HHS/United States
- S10 OD010707/OD/NIH HHS/United States
- R01 AR048852/AR/NIAMS NIH HHS/United States
- T32 DK108742/DK/NIDDK NIH HHS/United States
- R01 AR070864/AR/NIAMS NIH HHS/United States
- R01 AR069085/AR/NIAMS NIH HHS/United States
- P01 AR050245/AR/NIAMS NIH HHS/United States
- T32 GM007171/GM/NIGMS NIH HHS/United States
- R21 NS103007/NS/NINDS NIH HHS/United States
- T32 EB018266/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

