(Pro)renin receptor contributes to pregnancy-induced sodium-water retention in rats via activation of intrarenal RAAS and α-ENaC
- PMID: 30379098
- PMCID: PMC6459302
- DOI: 10.1152/ajprenal.00411.2018
(Pro)renin receptor contributes to pregnancy-induced sodium-water retention in rats via activation of intrarenal RAAS and α-ENaC
Abstract
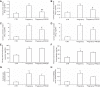
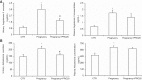
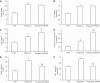
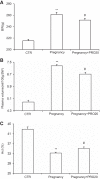
The (pro)renin receptor (PRR) is a new component of the renin-angiotensin-aldosterone system (RAAS) and regulates renin activity. The objective of the present study was to test potential roles of the renal PRR and intrarenal RAAS in the physiological status of late pregnancy. Late pregnant Sprague-Dawley rats were studied 19-21 days after sperm was observed in vaginal smears. Experiments were performed using age-matched virgin rats and late pregnant rats treated with the specific PRR inhibitor PRO20 (700 μg·kg-1·day-1 sc for 14 days, 3 times/day for every 8 h) or vehicle. The indices of RAAS, including PRR, renin, angiotensin II, and aldosterone levels, were examined by immunoblotting, qRT-PCR, or ELISA. Further analyses of renal epithelial sodium channel (ENaC) expression, sodium-water retention, and plasma volume were performed. We first present evidence for the activation of intrarenal RAAS in late pregnant rats, including increases in urinary renin activity, active and total renin content, and prorenin content, angiotensin II and aldosterone excretion, in parallel with increased renal PRR expression and urinary soluble PRR excretion. Functional evidence demonstrated that PRR antagonism with PRO20 effectively suppressed the indices of intrarenal RAAS in late pregnant rats. In addition, our results revealed that renal α-ENaC expression, sodium-water retention, and plasma volume were elevated during late pregnancy, which were all attenuated by PRO20. In summary, the present study examined the renal mechanism of sodium-water retention and plasma volume expansion in late pregnant rats and identified a novel role of PRR in regulation of intrarenal RAAS and α-ENaC and thus sodium and fluid retention associated with pregnancy.
Keywords: (pro)renin receptor; ENaC; plasma volume expansion; pregnancy; renin-angiotensin-aldosterone system.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
(Pro)renin Receptor Blockade Prevents Increases in Systolic Blood Pressure, Sodium Retention, and αENaC Protein Expression in the Kidney of 2K1C Goldblatt Mice.Int J Mol Sci. 2025 Apr 28;26(9):4177. doi: 10.3390/ijms26094177. Int J Mol Sci. 2025. PMID: 40362413 Free PMC article.
-
(Pro)Renin receptor regulates potassium homeostasis through a local mechanism.Am J Physiol Renal Physiol. 2017 Sep 1;313(3):F641-F656. doi: 10.1152/ajprenal.00043.2016. Epub 2016 Jul 20. Am J Physiol Renal Physiol. 2017. PMID: 27440776 Free PMC article.
-
(Pro)renin receptor contributes to regulation of renal epithelial sodium channel.J Hypertens. 2016 Mar;34(3):486-94; discussion 494. doi: 10.1097/HJH.0000000000000825. J Hypertens. 2016. PMID: 26771338 Free PMC article.
-
Soluble (pro)renin receptor as a novel regulator of renal medullary Na+ reabsorption.Am J Physiol Renal Physiol. 2025 Feb 1;328(2):F239-F247. doi: 10.1152/ajprenal.00156.2024. Epub 2024 Nov 7. Am J Physiol Renal Physiol. 2025. PMID: 39508841 Review.
-
Direct activation of ENaC by angiotensin II: recent advances and new insights.Curr Hypertens Rep. 2013 Feb;15(1):17-24. doi: 10.1007/s11906-012-0316-1. Curr Hypertens Rep. 2013. PMID: 23180052 Free PMC article. Review.
Cited by
-
(Pro)renin receptor decoy peptide PRO20 protects against adriamycin-induced nephropathy by targeting the intrarenal renin-angiotensin system.Am J Physiol Renal Physiol. 2020 Nov 1;319(5):F930-F940. doi: 10.1152/ajprenal.00279.2020. Epub 2020 Aug 31. Am J Physiol Renal Physiol. 2020. PMID: 32865014 Free PMC article.
-
Novel Antihypertensive Medications to Target the Renin-Angiotensin System: Mechanisms and Research.Rev Cardiovasc Med. 2025 Apr 21;26(4):27963. doi: 10.31083/RCM27963. eCollection 2025 Apr. Rev Cardiovasc Med. 2025. PMID: 40351692 Free PMC article. Review.
-
Activation of TRPC6 by AngⅡ Induces Podocyte Injury and Participates in Proteinuria of Nephrotic Syndrome.Front Pharmacol. 2022 Aug 3;13:915153. doi: 10.3389/fphar.2022.915153. eCollection 2022. Front Pharmacol. 2022. PMID: 35991898 Free PMC article.
-
UPLC-MS-Based Serum Metabolomics Reveals Potential Biomarkers of Ang II-Induced Hypertension in Mice.Front Cardiovasc Med. 2021 May 5;8:683859. doi: 10.3389/fcvm.2021.683859. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34026879 Free PMC article.
-
Importance of the (Pro)renin Receptor in Activating the Renin-Angiotensin System During Normotensive and Preeclamptic Pregnancies.Curr Hypertens Rep. 2024 Dec;26(12):483-495. doi: 10.1007/s11906-024-01316-1. Epub 2024 Aug 2. Curr Hypertens Rep. 2024. PMID: 39093387 Free PMC article. Review.
References
-
- Bharath LP, Cho JM, Park SK, Ruan T, Li Y, Mueller R, Bean T, Reese V, Richardson RS, Cai J, Sargsyan A, Pires K, Anandh Babu PV, Boudina S, Graham TE, Symons JD. Endothelial cell autophagy maintains shear stress-induced nitric oxide generation via glycolysis-dependent purinergic signaling to endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 37: 1646–1656, 2017. doi:10.1161/ATVBAHA.117.309510. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

