Thinking Outside the Box (and Arrow): Current Themes in Striatal Dysfunction in Movement Disorders
- PMID: 30379121
- PMCID: PMC6529282
- DOI: 10.1177/1073858418807887
Thinking Outside the Box (and Arrow): Current Themes in Striatal Dysfunction in Movement Disorders
Abstract
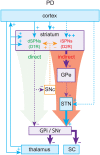
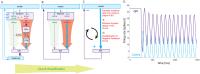
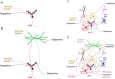
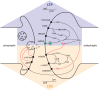
The basal ganglia are an intricately connected assembly of subcortical nuclei, forming the core of an adaptive network connecting cortical and thalamic circuits. For nearly three decades, researchers and medical practitioners have conceptualized how the basal ganglia circuit works, and how its pathology underlies motor disorders such as Parkinson's and Huntington's diseases, using what is often referred to as the "box-and-arrow model": a circuit diagram showing the broad strokes of basal ganglia connectivity and the pathological increases and decreases in the weights of specific connections that occur in disease. While this model still has great utility and has led to groundbreaking strategies to treat motor disorders, our evolving knowledge of basal ganglia function has made it clear that this classic model has several shortcomings that severely limit its predictive and descriptive abilities. In this review, we will focus on the striatum, the main input nucleus of the basal ganglia. We describe recent advances in our understanding of the rich microcircuitry and plastic capabilities of the striatum, factors not captured by the original box-and-arrow model, and provide examples of how such advances inform our current understanding of the circuit pathologies underlying motor disorders.
Keywords: direct and indirect pathway; dopamine acetylcholine balance; neurodegenerative diseases; striatal interneurons; striatal projection neurons; synaptic plasticity; synchronous oscillations.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

