A bacterial DNA repair pathway specific to a natural antibiotic
- PMID: 30379365
- PMCID: PMC6368877
- DOI: 10.1111/mmi.14158
A bacterial DNA repair pathway specific to a natural antibiotic
Abstract
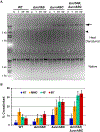
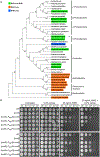
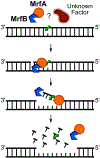
All organisms possess DNA repair pathways that are used to maintain the integrity of their genetic material. Although many DNA repair pathways are well understood, new pathways continue to be discovered. Here, we report an antibiotic specific DNA repair pathway in Bacillus subtilis that is composed of a previously uncharacterized helicase (mrfA) and exonuclease (mrfB). Deletion of mrfA and mrfB results in sensitivity to the DNA damaging agent mitomycin C, but not to any other type of DNA damage tested. We show that MrfAB function independent of canonical nucleotide excision repair, forming a novel excision repair pathway. We demonstrate that MrfB is a metal-dependent exonuclease and that the N-terminus of MrfB is required for interaction with MrfA. We determined that MrfAB failed to unhook interstrand cross-links in vivo, suggesting that MrfAB are specific to the monoadduct or the intrastrand cross-link. A phylogenetic analysis uncovered MrfAB homologs in diverse bacterial phyla, and cross-complementation indicates that MrfAB function is conserved in closely related species. B. subtilis is a soil dwelling organism and mitomycin C is a natural antibiotic produced by the soil bacterium Streptomyces lavendulae. The specificity of MrfAB suggests that these proteins are an adaptation to environments with mitomycin producing bacteria.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





Similar articles
-
Structural and biochemical characterization of the mitomycin C repair exonuclease MrfB.Nucleic Acids Res. 2024 Jun 24;52(11):6347-6359. doi: 10.1093/nar/gkae308. Nucleic Acids Res. 2024. PMID: 38661211 Free PMC article.
-
Structural and biochemical characterization of the mitomycin C repair exonuclease MrfB.bioRxiv [Preprint]. 2024 Feb 17:2024.02.15.580553. doi: 10.1101/2024.02.15.580553. bioRxiv. 2024. Update in: Nucleic Acids Res. 2024 Jun 24;52(11):6347-6359. doi: 10.1093/nar/gkae308. PMID: 38405983 Free PMC article. Updated. Preprint.
-
A conserved helicase motif of the AddA subunit of the Bacillus subtilis ATP-dependent nuclease (AddAB) is essential for DNA repair and recombination.Mol Microbiol. 1997 Jan;23(1):137-49. doi: 10.1046/j.1365-2958.1997.1991570.x. Mol Microbiol. 1997. PMID: 9004227
-
Bacterial DNA excision repair pathways.Nat Rev Microbiol. 2022 Aug;20(8):465-477. doi: 10.1038/s41579-022-00694-0. Epub 2022 Feb 24. Nat Rev Microbiol. 2022. PMID: 35210609 Free PMC article. Review.
-
Molecular mechanisms of DNA damage and repair: progress in plants.Crit Rev Biochem Mol Biol. 2001;36(4):337-97. doi: 10.1080/20014091074219. Crit Rev Biochem Mol Biol. 2001. PMID: 11563486 Review.
Cited by
-
RecA and RecB: probing complexes of DNA repair proteins with mitomycin C in live Escherichia coli with single-molecule sensitivity.J R Soc Interface. 2022 Aug;19(193):20220437. doi: 10.1098/rsif.2022.0437. Epub 2022 Aug 10. J R Soc Interface. 2022. PMID: 35946163 Free PMC article.
-
A deep dive into the RecQ interactome: something old and something new.Curr Genet. 2021 Oct;67(5):761-767. doi: 10.1007/s00294-021-01190-3. Epub 2021 May 7. Curr Genet. 2021. PMID: 33961099 Free PMC article. Review.
-
Quantitative proteomic reveals gallium maltolate induces an iron-limited stress response and reduced quorum-sensing in Pseudomonas aeruginosa.J Biol Inorg Chem. 2020 Dec;25(8):1153-1165. doi: 10.1007/s00775-020-01831-x. Epub 2020 Oct 30. J Biol Inorg Chem. 2020. PMID: 33125529
-
The yeast Hrq1 helicase stimulates Pso2 translesion nuclease activity and thereby promotes DNA interstrand crosslink repair.J Biol Chem. 2020 Jul 3;295(27):8945-8957. doi: 10.1074/jbc.RA120.013626. Epub 2020 May 5. J Biol Chem. 2020. PMID: 32371399 Free PMC article.
-
Structural and biochemical characterization of the mitomycin C repair exonuclease MrfB.Nucleic Acids Res. 2024 Jun 24;52(11):6347-6359. doi: 10.1093/nar/gkae308. Nucleic Acids Res. 2024. PMID: 38661211 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

