Trapping the Complex Molecular Machinery of Polyketide and Fatty Acid Synthases with Tunable Silylcyanohydrin Crosslinkers
- PMID: 30379389
- PMCID: PMC6407627
- DOI: 10.1002/anie.201806865
Trapping the Complex Molecular Machinery of Polyketide and Fatty Acid Synthases with Tunable Silylcyanohydrin Crosslinkers
Abstract
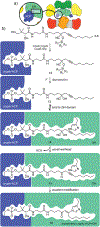
Many families of natural products are synthesized by large multidomain biological machines commonly referred to as megasynthases. While the advance of mechanism-based tools has opened new windows into the structural features within the protein-protein interfaces guiding carrier protein dependent enzymes, there is an immediate need for tools that can be engaged to link co-translated domains in a site-selective manner. Now, the use of silylcyanohydrins is demonstrated in a two-step, two-site selective crosslinking for the trapping of carrier-protein interactions within megasynthases. This advance provides a new tool to trap intermediate states within multimodular systems, a key step toward understanding the specificities within fatty acid (FAS) and polyketide (PKS) synthases.
Keywords: biosynthesis; natural products; protein crosslinking; protein labeling; synthases.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




Similar articles
-
The Structural Enzymology of Iterative Aromatic Polyketide Synthases: A Critical Comparison with Fatty Acid Synthases.Annu Rev Biochem. 2018 Jun 20;87:503-531. doi: 10.1146/annurev-biochem-063011-164509. Annu Rev Biochem. 2018. PMID: 29925265 Review.
-
Clearing the skies over modular polyketide synthases.ACS Chem Biol. 2006 Sep 19;1(8):505-9. doi: 10.1021/cb600376r. ACS Chem Biol. 2006. PMID: 17168537
-
Computational structural enzymology methodologies for the study and engineering of fatty acid synthases, polyketide synthases and nonribosomal peptide synthetases.Methods Enzymol. 2019;622:375-409. doi: 10.1016/bs.mie.2019.03.001. Epub 2019 Apr 22. Methods Enzymol. 2019. PMID: 31155062 Free PMC article.
-
Structural enzymology of polyketide synthases.Methods Enzymol. 2009;459:17-47. doi: 10.1016/S0076-6879(09)04602-3. Methods Enzymol. 2009. PMID: 19362634 Free PMC article.
-
The chemical biology of modular biosynthetic enzymes.Chem Soc Rev. 2009 Jul;38(7):2012-45. doi: 10.1039/b805115c. Epub 2009 Mar 27. Chem Soc Rev. 2009. PMID: 19551180 Review.
Cited by
-
Probing the structure and function of acyl carrier proteins to unlock the strategic redesign of type II polyketide biosynthetic pathways.J Biol Chem. 2021 Jan-Jun;296:100328. doi: 10.1016/j.jbc.2021.100328. Epub 2021 Jan 23. J Biol Chem. 2021. PMID: 33493513 Free PMC article. Review.
-
Visualizing acyl carrier protein interactions within a crosslinked type I polyketide synthase.Nat Commun. 2025 Aug 21;16(1):7798. doi: 10.1038/s41467-025-63024-x. Nat Commun. 2025. PMID: 40841798 Free PMC article.
-
Masked cerulenin enables a dual-site selective protein crosslink.Chem Sci. 2023 Sep 8;14(39):10925-10933. doi: 10.1039/d3sc02864j. eCollection 2023 Oct 11. Chem Sci. 2023. PMID: 37829009 Free PMC article.
-
Developing crosslinkers specific for epimerization domain in NRPS initiation modules to evaluate mechanism.RSC Chem Biol. 2022 Jan 27;3(3):312-319. doi: 10.1039/d2cb00005a. eCollection 2022 Mar 9. RSC Chem Biol. 2022. PMID: 35359491 Free PMC article.
-
Fluorometric Analysis of Carrier-Protein-Dependent Biosynthesis through a Conformationally Sensitive Solvatochromic Pantetheinamide Probe.ACS Chem Biol. 2024 Jul 19;19(7):1416-1425. doi: 10.1021/acschembio.4c00169. Epub 2024 Jun 23. ACS Chem Biol. 2024. PMID: 38909314 Free PMC article.
References
-
- a) Artigues A, Nadeau OW, Rimmer MA, Villar MT, Du X, Fenton AW, Carlson GM, Adv. Exp. Med. Biol 2016, 919, 397–431; - PMC - PubMed
- b) Sinz A, Arlt C, Chorev D, Sharon M, Protein Sci 2015, 24, 1193–1209; - PMC - PubMed
- c) Heck T, Faccio G, Richter M, Thöny-Meyer L, Appl. Microbiol. Biotechnol 2013, 97, 461–475. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

