A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair
- PMID: 30379414
- PMCID: PMC6521871
- DOI: 10.1002/adhm.201800672
A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair
Abstract
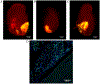
Congenital heart defects are present in 8 of 1000 newborns and palliative surgical therapy has increased survival. Despite improved outcomes, many children develop reduced cardiac function and heart failure requiring transplantation. Human cardiac progenitor cell (hCPC) therapy has potential to repair the pediatric myocardium through release of reparative factors, but therapy suffers from limited hCPC retention and functionality. Decellularized cardiac extracellular matrix hydrogel (cECM) improves heart function in animals, and human trials are ongoing. In the present study, a 3D-bioprinted patch containing cECM for delivery of pediatric hCPCs is developed. Cardiac patches are printed with bioinks composed of cECM, hCPCs, and gelatin methacrylate (GelMA). GelMA-cECM bioinks print uniformly with a homogeneous distribution of cECM and hCPCs. hCPCs maintain >75% viability and incorporation of cECM within patches results in a 30-fold increase in cardiogenic gene expression of hCPCs compared to hCPCs grown in pure GelMA patches. Conditioned media from GelMA-cECM patches show increased angiogenic potential (>2-fold) over GelMA alone, as seen by improved endothelial cell tube formation. Finally, patches are retained on rat hearts and show vascularization over 14 d in vivo. This work shows the successful bioprinting and implementation of cECM-hCPC patches for potential use in repairing damaged myocardium.
Keywords: bioprinting; cardiac extracellular matrix; cardiac patches; cardiac progenitor cells; pediatric heart failure.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






References
-
- Rossano JW, Kim JJ, Decker JA, Price JF, Zafar F, Graves DE, Morales DL, Heinle JS, Bozkurt B, Denfield SW, Circulation. 2010, 122, A1374.
-
- Robbins JM, Bird TM, Tilford JM, Cleves MA, Hobbs CA, Grosse SD, Correa A, MMWR. 2007, 56, 25. - PubMed
-
- Bove EL, Pediatr. Cardiol. 1998, 19, 308. - PubMed
-
- Segers VF, Lee RT, Nature 2008, 451, 937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

