Acoustogenic Probes: A New Frontier in Photoacoustic Imaging
- PMID: 30379532
- PMCID: PMC7303888
- DOI: 10.1021/acs.accounts.8b00351
Acoustogenic Probes: A New Frontier in Photoacoustic Imaging
Abstract
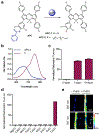
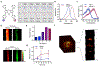
Photoacoustic imaging (PAI) is a powerful imaging modality capable of mapping the absorption of light in biological tissue via the PA effect. When chromophores are optically excited, subsequent energy loss in the form of heat generates local thermoelastic expansion. Repeated excitation from a pulsed laser induces pressure fluctuations that propagate through tissue and can be detected as ultrasound waves. By combining ultrasonic detection with optical excitation, PAI enables high-resolution image acquisition at centimeter depths. PAI is also relatively inexpensive and relies on safe, nonionizing excitation light in the near-infrared window, making it an attractive alternative to other common biomedical imaging modalities. Research in our group is aimed at developing small-molecule activatable probes that can be used for analyte detection in deep tissue via PAI. These probes contain reactive triggers that undergo a selective chemical reaction in the presence of specific stimuli to produce a spectral change that can be observed via PAI. Chemically tuning the absorbance profile of the probe and the reacted product such that they are both within the PA imaging window enables ratiometric imaging when each species is irradiated at a specific wavelength. Ratiometric imaging is an important design feature of these probes as it minimizes error associated with tissue-dependent signal fluctuations and instrumental variation. In this Account, we discuss key properties for designing small-molecule PA probes that can be applied for in vivo studies and the challenges associated with this area of probe development. We also highlight examples from our group including probes capable of detecting metal ions (Cu(II)), reactive nitrogen species (NO), and oxygen tension (hypoxia). Each of these targets can be sensed using a modular design strategy based on influencing the electronic and spectral properties of a NIR-absorbing dye platform. We demonstrate that ideal small-molecule PA probes have high molar absorptivity, low fluorescence quantum yields, and selective triggers that can reliably report on a single analyte in a complex biological setting. Probes must also be highly chemo- and photostable to enable long-term imaging studies. We show that these PA probes react rapidly and selectively and can be utilized for deep-tissue imaging in mouse models of various diseases. Overall, these examples represent a new class of biomedical imaging tools that seek to enable high-resolution molecular imaging capable of improving diagnostic methods and elucidating new biological discoveries. We anticipate that the combination of small-molecule PA probes with new PAI technology will enable noninvasive detection of analytes relevant to disease progression and mapping of tissue microenvironments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


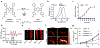

References
-
- James ML; Gambhir SS A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications. Physiol. Rev 2012, 92, 897–965. - PubMed
-
- Grimm JB; Heckman LM; Lavis LD The Chemistry of Small-Molecule Fluorogenic Probes. Prog. Mol. Biol. Transl. Sci 2013, 113, 1–34. - PubMed
-
- Ntziachristos V Going Deeper than Microscopy: The Optical Imaging Frontier in Biology. Nat. Methods 2010, 7, 603–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

