Bromine inhalation mimics ischemia-reperfusion cardiomyocyte injury and calpain activation in rats
- PMID: 30379573
- PMCID: PMC6383353
- DOI: 10.1152/ajpheart.00652.2017
Bromine inhalation mimics ischemia-reperfusion cardiomyocyte injury and calpain activation in rats
Abstract
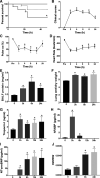
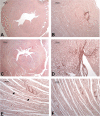
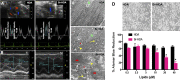
Halogens are widely used, highly toxic chemicals that pose a potential threat to humans because of their abundance. Halogens such as bromine (Br2) cause severe pulmonary and systemic injuries; however, the mechanisms of their toxicity are largely unknown. Here, we demonstrated that Br2 and reactive brominated species produced in the lung and released in blood reach the heart and cause acute cardiac ultrastructural damage and dysfunction in rats. Br2-induced cardiac damage was demonstrated by acute (3-24 h) increases in circulating troponin I, heart-type fatty acid-binding protein, and NH2-terminal pro-brain natriuretic peptide. Transmission electron microscopy demonstrated acute (3-24 h) cardiac contraction band necrosis, disruption of z-disks, and mitochondrial swelling and disorganization. Echocardiography and hemodynamic analysis revealed left ventricular (LV) systolic and diastolic dysfunction at 7 days. Plasma and LV tissue had increased levels of brominated fatty acids. 2-Bromohexadecanal (Br-HDA) injected into the LV cavity of a normal rat caused acute LV enlargement with extensive disruption of the sarcomeric architecture and mitochondrial damage. There was extensive infiltration of neutrophils and increased myeloperoxidase levels in the hearts of Br2- or Br2 reactant-exposed rats. Increased bromination of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) and increased phosphalamban after Br2 inhalation decreased cardiac SERCA activity by 70%. SERCA inactivation was accompanied by increased Ca2+-sensitive LV calpain activity. The calpain-specific inhibitor MDL28170 administered within 1 h after exposure significantly decreased calpain activity and acute mortality. Bromine inhalation and formation of reactive brominated species caused acute cardiac injury and myocardial damage that can lead to heart failure. NEW & NOTEWORTHY The present study defines left ventricular systolic and diastolic dysfunction due to cardiac injury after bromine (Br2) inhalation. A calpain-dependent mechanism was identified as a potential mediator of cardiac ultrastructure damage. This study not only highlights the importance of monitoring acute cardiac symptoms in victims of Br2 exposure but also defines calpains as a potential target to treat Br2-induced toxicity.
Keywords: calcium; calpains; cardiac; halogen; neutrophil; sarco(endo)plasmic reticulum Ca-ATPase.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Ahmad S, Ahmad A, Hendry-Hofer TB, Loader JE, Claycomb WC, Mozziconacci O, Schöneich C, Reisdorph N, Powell RL, Chandler JD, Day BJ, Veress LA, White CW. Sarcoendoplasmic reticulum Ca2+ ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. Am J Respir Cell Mol Biol 52: 492–502, 2015. doi: 10.1165/rcmb.2014-0005OC. - DOI - PMC - PubMed
-
- Ahmed MI, Guichard JL, Soorappan RN, Ahmad S, Mariappan N, Litovsky S, Gupta H, Lloyd SG, Denney TS, Powell PC, Aban I, Collawn J, Davies JE, McGiffin DC, Dell’Italia LJ. Disruption of desmin-mitochondrial architecture in patients with regurgitant mitral valves and preserved ventricular function. J Thorac Cardiovasc Surg 152: 1059–1070.e2, 2016. doi: 10.1016/j.jtcvs.2016.06.017. - DOI - PMC - PubMed
-
- Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive brominating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: disparate utilization of sodium halides in the production of alpha-halo fatty aldehydes. J Biol Chem 277: 4694–4703, 2002. doi: 10.1074/jbc.M110875200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

