Tuft Cells-Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry
- PMID: 30379593
- PMCID: PMC8352721
- DOI: 10.1146/annurev-immunol-042718-041505
Tuft Cells-Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry
Abstract
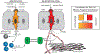
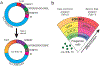
Tuft cells-rare solitary chemosensory cells in mucosal epithelia-are undergoing intense scientific scrutiny fueled by recent discovery of unsuspected connections to type 2 immunity. These cells constitute a conduit by which ligands from the external space are sensed via taste-like signaling pathways to generate outputs unique among epithelial cells: the cytokine IL-25, eicosanoids associated with allergic immunity, and the neurotransmitter acetylcholine. The classic type II taste cell transcription factor POU2F3 is lineage defining, suggesting a conceptualization of these cells as widely distributed environmental sensors with effector functions interfacing type 2 immunity and neural circuits. Increasingly refined single-cell analytics have revealed diversity among tuft cells that extends from nasal epithelia and type II taste cells to ex-Aire-expressing medullary thymic cells and small-intestine cells that mediate tissue remodeling in response to colonizing helminths and protists.
Keywords: IL-25; POU2F3; TRPM5; chemosensation; tuft cells; type 2 immunity.
Conflict of interest statement
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Figures

Similar articles
-
Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system.Mucosal Immunol. 2016 Nov;9(6):1353-1359. doi: 10.1038/mi.2016.68. Epub 2016 Aug 24. Mucosal Immunol. 2016. PMID: 27554294 Review.
-
Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites.Nature. 2016 Jan 14;529(7585):226-30. doi: 10.1038/nature16527. Nature. 2016. PMID: 26762460 Free PMC article.
-
Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice.PLoS One. 2017 Dec 7;12(12):e0189340. doi: 10.1371/journal.pone.0189340. eCollection 2017. PLoS One. 2017. PMID: 29216297 Free PMC article.
-
Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut.Science. 2016 Mar 18;351(6279):1329-33. doi: 10.1126/science.aaf1648. Epub 2016 Feb 4. Science. 2016. PMID: 26847546 Free PMC article.
-
Helminth Sensing at the Intestinal Epithelial Barrier-A Taste of Things to Come.Front Immunol. 2020 Jul 30;11:1489. doi: 10.3389/fimmu.2020.01489. eCollection 2020. Front Immunol. 2020. PMID: 32849506 Free PMC article. Review.
Cited by
-
Tumor suppressor p53 regulates intestinal type 2 immunity.Nat Commun. 2021 Jun 7;12(1):3371. doi: 10.1038/s41467-021-23587-x. Nat Commun. 2021. PMID: 34099671 Free PMC article.
-
Epithelial Cells as a Transmitter of Signals From Commensal Bacteria and Host Immune Cells.Front Immunol. 2019 Aug 28;10:2057. doi: 10.3389/fimmu.2019.02057. eCollection 2019. Front Immunol. 2019. PMID: 31555282 Free PMC article. Review.
-
A Proximal-to-Distal Survey of Healthy Adult Human Small Intestine and Colon Epithelium by Single-Cell Transcriptomics.Cell Mol Gastroenterol Hepatol. 2022;13(5):1554-1589. doi: 10.1016/j.jcmgh.2022.02.007. Epub 2022 Feb 15. Cell Mol Gastroenterol Hepatol. 2022. PMID: 35176508 Free PMC article.
-
New insights into tuft cell formation: Implications for structure-function relationships.Curr Opin Cell Biol. 2022 Jun;76:102082. doi: 10.1016/j.ceb.2022.102082. Epub 2022 Apr 22. Curr Opin Cell Biol. 2022. PMID: 35468541 Free PMC article. Review.
-
Altered peripheral taste function in a mouse model of inflammatory bowel disease.Sci Rep. 2023 Nov 2;13(1):18895. doi: 10.1038/s41598-023-46244-3. Sci Rep. 2023. PMID: 37919307 Free PMC article.
References
-
- Sato A 2007. Tuft cells. Anat Sci Int 82: 187–99 - PubMed
-
- von Moltke J 2018. Chapter 31 - Intestinal Tuft Cells A2 - Said, Hamid M In Physiology of the Gastrointestinal Tract (Sixth Edition), pp. 721–33: Academic Press

