Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation
- PMID: 30379623
- PMCID: PMC6442925
- DOI: 10.1152/physrev.00036.2017
Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation
Abstract
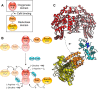
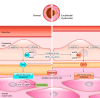
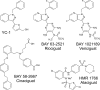
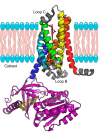
Nitric oxide (NO) is a small free radical with critical signaling roles in physiology and pathophysiology. The generation of sufficient NO levels to regulate the resistance of the blood vessels and hence the maintenance of adequate blood flow is critical to the healthy performance of the vasculature. A novel paradigm indicates that classical NO synthesis by dedicated NO synthases is supplemented by nitrite reduction pathways under hypoxia. At the same time, reactive oxygen species (ROS), which include superoxide and hydrogen peroxide, are produced in the vascular system for signaling purposes, as effectors of the immune response, or as byproducts of cellular metabolism. NO and ROS can be generated by distinct enzymes or by the same enzyme through alternate reduction and oxidation processes. The latter oxidoreductase systems include NO synthases, molybdopterin enzymes, and hemoglobins, which can form superoxide by reduction of molecular oxygen or NO by reduction of inorganic nitrite. Enzymatic uncoupling, changes in oxygen tension, and the concentration of coenzymes and reductants can modulate the NO/ROS production from these oxidoreductases and determine the redox balance in health and disease. The dysregulation of the mechanisms involved in the generation of NO and ROS is an important cause of cardiovascular disease and target for therapy. In this review we will present the biology of NO and ROS in the cardiovascular system, with special emphasis on their routes of formation and regulation, as well as the therapeutic challenges and opportunities for the management of NO and ROS in cardiovascular disease.
Conflict of interest statement
M.T. Gladwin is a coinventor on a National Institutes of Health government patent application for the use of nitrite salts in the treatment of cardiovascular diseases. J. Tejero and S. Shiva do not declare any conflicts of interest, financial or otherwise.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

