Occurrence of Treatment-Related Cardiotoxicity and Its Impact on Outcomes Among Children Treated in the AAML0531 Clinical Trial: A Report From the Children's Oncology Group
- PMID: 30379624
- PMCID: PMC6354770
- DOI: 10.1200/JCO.18.00313
Occurrence of Treatment-Related Cardiotoxicity and Its Impact on Outcomes Among Children Treated in the AAML0531 Clinical Trial: A Report From the Children's Oncology Group
Abstract
Purpose: Late cardiotoxicity after pediatric acute myeloid leukemia therapy causes substantial morbidity and mortality. The impact of early-onset cardiotoxicity on treatment outcomes is less well understood. Thus, we evaluated the risk factors for incident early cardiotoxicity and the impacts of cardiotoxicity on event-free survival (EFS) and overall survival (OS).
Methods: Cardiotoxicity was ascertained through adverse event monitoring over the course of follow-up among 1,022 pediatric patients with acute myeloid leukemia treated in the Children's Oncology Group trial AAML0531. It was defined as grade 2 or higher left ventricular systolic dysfunction on the basis of Common Terminology Criteria for Adverse Events (version 3) definitions.
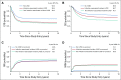
Results: Approximately 12% of patients experienced cardiotoxicity over a 5-year follow-up, with more than 70% of incident events occurring during on-protocol therapy. Documented cardiotoxicity during on-protocol therapy was significantly associated with subsequent off-protocol toxicity. Overall, the incidence was higher among noninfants and black patients, and in the setting of a bloodstream infection. Both EFS (hazard ratio [HR], 1.6; 95% CI, 1.2 to 2.1; P = .004) and OS (HR, 1.6; 95% CI, 1.2 to 2.2, P = .005) were significantly worse in patients with documented cardiotoxicity. Impacts on EFS were equivalent whether the incident cardiotoxicity event occurred in the absence (HR, 1.6; 95% CI, 1.1 to 2.2; P = .017) or presence of infection (HR, 1.6; 95% CI, 1.0 to 2.7; P = .069) compared with patients without documented cardiotoxicity. However, the reduction in OS was more pronounced for cardiotoxicity not associated with infection (HR, 1.7; 95% CI, 1.2 to 2.5; P = .004) than for infection-associated cardiotoxicity (HR, 1.3; 95% CI, 0.7 to 2.4; P = .387).
Conclusion: Early treatment-related cardiotoxicity may be associated with decreased EFS and OS. Cardioprotective strategies are urgently needed to improve relapse risk and both short- and long-term mortality outcomes.
Figures
Comment in
-
Optimizing Cardiovascular Care in Children With Acute Myeloid Leukemia to Improve Cancer-Related Outcomes.J Clin Oncol. 2019 Jan 1;37(1):1-6. doi: 10.1200/JCO.18.01421. Epub 2018 Nov 13. J Clin Oncol. 2019. PMID: 30422740
References
-
- Faulk K, Gore L, Cooper T: Overview of therapy and strategies for optimizing outcomes in de novo pediatric acute myeloid leukemia. Paediatr Drugs 16:213-227, 2014 - PubMed
-
- Gamis AS, Alonzo TA, Meshinchi S, et al. : Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol 32:3021-3032, 2014 - PMC - PubMed
-
- Kaspers GJ, Creutzig U: Pediatric acute myeloid leukemia: International progress and future directions. Leukemia 19:2025-2029, 2005 - PubMed
-
- Rubnitz JE: Current management of childhood acute myeloid leukemia. Paediatr Drugs 19:1-10, 2017 - PubMed
-
- Aplenc R, Meshinchi S, Sung L, et al. : The addition of bortezomib to standard chemotherapy for pediatric acute myeloid leukemia has increased toxicity without therapeutic benefit: A report from the Children’s Oncology Group. Blood 128:899, 2016
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical