Activity profiling of peptidases in Angiostrongylus costaricensis first-stage larvae and adult worms
- PMID: 30379807
- PMCID: PMC6231675
- DOI: 10.1371/journal.pntd.0006923
Activity profiling of peptidases in Angiostrongylus costaricensis first-stage larvae and adult worms
Abstract
Background: Angiostrongylus costaricensis is a relatively uncharacterized nematode that causes abdominal angiostrongyliasis in Latin America, a human parasitic disease. Currently, no effective pharmacological treatment for angiostrongyliasis exists. Peptidases are known to be druggable targets for a variety of diseases and are essential for several biological processes in parasites. Therefore, this study aimed to systematically characterize the peptidase activity of A. costaricensis in different developmental stages of this parasitic nematode.
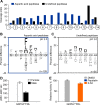
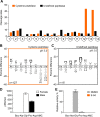
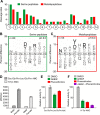
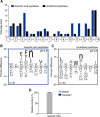
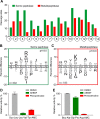
Methodology/principal findings: A library of diverse tetradecapeptides was incubated with cellular lysates from adult worms and from first-stage larvae (L1) and cleaved peptide products were identified by mass spectrometry. Lysates were also treated with class specific peptidase inhibitors to determine which enzyme class was responsible for the proteolytic activity. Peptidase activity from the four major mechanistic classes (aspartic, metallo, serine and cysteine) were detected in adult worm lysate, whereas aspartic, metallo and serine-peptidases were found in the larval lysates. In addition, the substrate specificity profile was found to vary at different pH values.
Conclusions/significance: The proteolytic activities in adult worm and L1 lysates were characterized using a highly diversified library of peptide substrates and the activity was validated using a selection of fluorescent substrates. Taken together, peptidase signatures for different developmental stages of this parasite has improved our understanding of the disease pathogenesis and may be useful as potential drug targets or vaccine candidates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Abdominal angiostrongyliasis in the Americas: fifty years since the discovery of a new metastrongylid species, Angiostrongylus costaricensis.Parasit Vectors. 2021 Jul 22;14(1):374. doi: 10.1186/s13071-021-04875-3. Parasit Vectors. 2021. PMID: 34294132 Free PMC article. Review.
-
Proteolytic activity in the adult and larval stages of the human roundworm parasite Angiostrongylus costaricensis.Mem Inst Oswaldo Cruz. 2012 Sep;107(6):752-9. doi: 10.1590/s0074-02762012000600008. Mem Inst Oswaldo Cruz. 2012. PMID: 22990964
-
[Human angiostrongyliasis caused by Angiostrongylus costaricensis].Bull Acad Natl Med. 1994 Apr;178(4):625-31; discussion 632-3. Bull Acad Natl Med. 1994. PMID: 8076197 Review. French.
-
Comprehensive proteomic profiling of adult Angiostrongylus costaricensis, a human parasitic nematode.J Proteomics. 2011 Aug 24;74(9):1545-59. doi: 10.1016/j.jprot.2011.04.031. Epub 2011 May 10. J Proteomics. 2011. PMID: 21596163
-
Proteolytic activity in Hysterothylacium aduncum (Nematoda: Anisakidae), a fish gastrointestinal parasite of worldwide distribution.Vet Parasitol. 2011 Dec 29;183(1-2):95-102. doi: 10.1016/j.vetpar.2011.07.002. Epub 2011 Jul 12. Vet Parasitol. 2011. PMID: 21802207
Cited by
-
A comparative 'omics' approach for prediction of candidate Strongyloides stercoralis diagnostic coproantigens.PLoS Negl Trop Dis. 2023 Apr 17;17(4):e0010777. doi: 10.1371/journal.pntd.0010777. eCollection 2023 Apr. PLoS Negl Trop Dis. 2023. PMID: 37068106 Free PMC article.
-
Abdominal angiostrongyliasis in the Americas: fifty years since the discovery of a new metastrongylid species, Angiostrongylus costaricensis.Parasit Vectors. 2021 Jul 22;14(1):374. doi: 10.1186/s13071-021-04875-3. Parasit Vectors. 2021. PMID: 34294132 Free PMC article. Review.
-
Multiplex substrate profiling by mass spectrometry for proteases.Methods Enzymol. 2023;682:375-411. doi: 10.1016/bs.mie.2022.09.009. Epub 2022 Dec 21. Methods Enzymol. 2023. PMID: 36948708 Free PMC article.
References
-
- Morera P, Cespedes R. Angiostrongilosis abdominal. Uma nueva parasitosis humana. Acta Med Costarric. 1971;(14):173–89.
-
- Romero-Alegría A, Belhassen-García M, Velasco-Tirado V, Garcia-Mingo A, Alvela-Suárez L, Pardo-Lledias J, et al. Angiostrongylus costaricensis: systematic review of case reports. Advances in Infectious Diseases 2014;4:36–41. 10.4236/aid.2014.41007 - DOI
-
- Morera P. Life history and redescription of Angiostrongylus costaricensis Morera and Cespedes, 1971. Am J Trop Med Hyg. 1973;22(5):613–21. . - PubMed
-
- Thiengo SC. Mode of Infection of Sarasinula marginata (Mollusca) with Larvae of Angiostrongylus costaricensis (Nematoda). Mem Inst Oswaldo Cruz. 1996;91 (3):277–8. 10.1590/S0074-02761996 - DOI

