Recording and reproducing the diurnal oviposition rhythms of wild populations of the soft- and stone- fruit pest Drosophila suzukii
- PMID: 30379809
- PMCID: PMC6209131
- DOI: 10.1371/journal.pone.0199406
Recording and reproducing the diurnal oviposition rhythms of wild populations of the soft- and stone- fruit pest Drosophila suzukii
Abstract
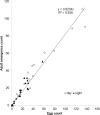
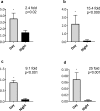
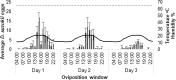
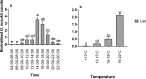
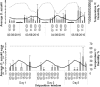
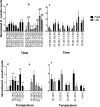
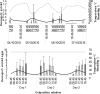
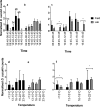
Drosophila suzukii is a horticultural pest on a global scale which causes both yield and economic losses on a range of soft- and stone-fruit. Tackling this pest is problematic but exploiting behavioral rhythms could increase the impact of control. To do this, a better understanding of behavioral patterns is needed. Within this study we aimed to investigate rhythms in reproductive behavior of wild D. suzukii under natural conditions in the field. Environmental parameters were also recorded to decipher how they influence these rhythms. Assays were then performed on laboratory cultures, housed under artificial conditions mimicking the temperature and light cycles, to see if these patterns were reproducible and rhythmic. We were able to promote field like oviposition patterns within the laboratory using realistic temperature and light cycles regardless of variations in other factors including substrate, humidity, and lighting type. Locomotion activity was also recorded under these mimicked conditions to identify how this behavior interacts with oviposition rhythms. Both our field and laboratory assays show that oviposition behavior is likely under the control of the circadian clock and primarily occurs during the day. However, consistent with prior reports we observed that these patterns become crepuscular when day-time temperature peaks exceeded 30°C. This was also found within locomotion rhythms. With an increased understanding of how these behaviors are influenced by environmental conditions, we highlight the importance of using realistic temperature and light cycles when investigating behavioral patterns. From an increased understanding of D. suzukii behavior we increase our ability to target the pest in the field.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Control of Daily Locomotor Activity Patterns in Drosophila suzukii by the Circadian Clock, Light, Temperature and Social Interactions.J Biol Rhythms. 2019 Oct;34(5):463-481. doi: 10.1177/0748730419869085. Epub 2019 Aug 22. J Biol Rhythms. 2019. PMID: 31436123
-
Reducing Drosophila suzukii emergence through inter-species competition.Pest Manag Sci. 2018 Jun;74(6):1466-1471. doi: 10.1002/ps.4836. Epub 2018 Feb 20. Pest Manag Sci. 2018. PMID: 29266721
-
Behavioral response of spotted-wing drosophila, Drosophila suzukii Matsumura, to aversive odors and a potential oviposition deterrent in the field.Pest Manag Sci. 2016 Apr;72(4):701-6. doi: 10.1002/ps.4040. Epub 2015 Jun 12. Pest Manag Sci. 2016. PMID: 25973596
-
Factors influencing oviposition behaviour of the invasive pest, Drosophila suzukii, derived from interactions with other Drosophila species: potential applications for control.Pest Manag Sci. 2023 Nov;79(11):4132-4139. doi: 10.1002/ps.7693. Epub 2023 Aug 8. Pest Manag Sci. 2023. PMID: 37516913 Free PMC article. Review.
-
Advances in the Chemical Ecology of the Spotted Wing Drosophila (Drosophila suzukii) and its Applications.J Chem Ecol. 2018 Oct;44(10):922-939. doi: 10.1007/s10886-018-1000-y. Epub 2018 Jul 27. J Chem Ecol. 2018. PMID: 30054769 Review.
Cited by
-
Locomotor Behaviour and Clock Neurons Organisation in the Agricultural Pest Drosophila suzukii.Front Physiol. 2019 Jul 24;10:941. doi: 10.3389/fphys.2019.00941. eCollection 2019. Front Physiol. 2019. PMID: 31396106 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

