Suppression of miR-22, a tumor suppressor in cervical cancer, by human papillomavirus 16 E6 via a p53/miR-22/HDAC6 pathway
- PMID: 30379969
- PMCID: PMC6209303
- DOI: 10.1371/journal.pone.0206644
Suppression of miR-22, a tumor suppressor in cervical cancer, by human papillomavirus 16 E6 via a p53/miR-22/HDAC6 pathway
Abstract
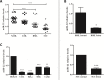
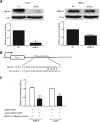
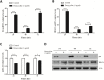
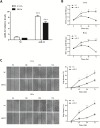
MicroRNAs (miRNAs) are small non-coding RNAs that function to down-regulate gene expression involving in various cellular processes related to carcinogenesis. Recently, miR-22 was identified as a tumor-suppressing miRNA in many human cancers. However, the regulatory mechanism and the specific function of this miRNA in cervical cancer remain unclear. In the present study, we carried out gene transfection, western blot and quantitative RT-PCR to explore the regulatory mechanism and the functional role of miR-22 in cervical cancer. We verified that miR-22 was down-regulated in cervical cancer tissues and cervical cancer cell lines relative to matched non-tumor tissues and normal human cervical keratinocyte line (HCK1T). By contrast, histone deacetylase 6 (HDAC6) was inversely correlated with miR-22 in both cervical tissues and cancer cell lines. Mechanically, HDAC6 was down-regulated by miR-22 at the post-transcriptional level, via a specific target site within the 3'UTR, identified by a luciferase reporter assay. Moreover, we also showed that the correlation between miR-22 and HDAC6 expression was regulated by an E6/p53 pathway in HCK1Ts expressing HPV16 E6. For functional study, an ectopic expression of miR-22 could inhibit cell proliferation and migration, and could induce apoptosis of cervical cancer cell lines. These findings demonstrated that miR-22 was down-regulated in cervical cancer and inversely collated with its downstream target HDAC6. MiR-22 acts as tumor suppressor by inhibiting proliferation and migration, and by inducing apoptosis of cervical cancer cell lines by targeting the 3'UTR of HDAC6. This newly identified E6/p53/miR-22/HDAC6 regulatory network might be a candidate therapeutic target for cervical cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
MicroRNA-331-3p Suppresses Cervical Cancer Cell Proliferation and E6/E7 Expression by Targeting NRP2.Int J Mol Sci. 2016 Aug 18;17(8):1351. doi: 10.3390/ijms17081351. Int J Mol Sci. 2016. PMID: 27548144 Free PMC article.
-
miR-2861 acts as a tumor suppressor via targeting EGFR/AKT2/CCND1 pathway in cervical cancer induced by human papillomavirus virus 16 E6.Sci Rep. 2016 Jul 1;6:28968. doi: 10.1038/srep28968. Sci Rep. 2016. PMID: 27364926 Free PMC article.
-
Recombinant adenoviruses expressing HPV16/18 E7 upregulate the HDAC6 and DNMT3B genes in C33A cells.Front Cell Infect Microbiol. 2024 Oct 1;14:1459572. doi: 10.3389/fcimb.2024.1459572. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39411320 Free PMC article.
-
Regulation of cell cycle progression and apoptosis by the papillomavirus E6 oncogene.Crit Rev Eukaryot Gene Expr. 2004;14(3):183-202. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.30. Crit Rev Eukaryot Gene Expr. 2004. PMID: 15248815 Review.
-
Interactive functions of microRNAs in the miR-23a-27a-24-2 cluster and the potential for targeted therapy in cancer.J Cell Physiol. 2020 Jan;235(1):6-16. doi: 10.1002/jcp.28958. Epub 2019 Jun 13. J Cell Physiol. 2020. PMID: 31192453 Review.
Cited by
-
The Dysregulation of MicroRNAs in the Development of Cervical Pre-Cancer-An Update.Int J Mol Sci. 2022 Jun 27;23(13):7126. doi: 10.3390/ijms23137126. Int J Mol Sci. 2022. PMID: 35806128 Free PMC article. Review.
-
MicroRNA-22 represses glioma development via activation of macrophage-mediated innate and adaptive immune responses.Oncogene. 2022 Apr;41(17):2444-2457. doi: 10.1038/s41388-022-02236-7. Epub 2022 Mar 12. Oncogene. 2022. PMID: 35279703
-
The complexity of human papilloma virus in cancers: a narrative review.Infect Agent Cancer. 2023 Feb 26;18(1):13. doi: 10.1186/s13027-023-00488-w. Infect Agent Cancer. 2023. PMID: 36843070 Free PMC article. Review.
-
MicroRNA Post-transcriptional Regulation of the NLRP3 Inflammasome in Immunopathologies.Front Pharmacol. 2019 May 1;10:451. doi: 10.3389/fphar.2019.00451. eCollection 2019. Front Pharmacol. 2019. PMID: 31118894 Free PMC article. Review.
-
Proteomics Analysis of Andrographolide-Induced Apoptosis via the Regulation of Tumor Suppressor p53 Proteolysis in Cervical Cancer-Derived Human Papillomavirus 16-Positive Cell Lines.Int J Mol Sci. 2021 Jun 24;22(13):6806. doi: 10.3390/ijms22136806. Int J Mol Sci. 2021. PMID: 34202736 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

