Differential Roles of IL-2 Signaling in Developing versus Mature Tregs
- PMID: 30380412
- PMCID: PMC6289175
- DOI: 10.1016/j.celrep.2018.10.002
Differential Roles of IL-2 Signaling in Developing versus Mature Tregs
Abstract
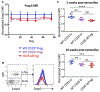
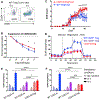
Although Foxp3+ regulatory T cells (Tregs) require interleukin-2 (IL-2) for their development, it has been unclear whether continuing IL-2 signals are needed to maintain lineage stability, survival, and suppressor function in mature Tregs. We generated mice in which CD25, the main ligand-binding subunit of the IL-2 receptor, can be inducibly deleted from Tregs after thymic development. In contrast to Treg development, we find that IL-2 is dispensable for maintaining lineage stability in mature Tregs. Although continuous IL-2 signaling is needed for long-term Treg survival, CD25-deleted Tregs may persist for several weeks in vivo using IL-7. We also observe defects in glycolytic metabolism and suppressor function following CD25 deletion. Thus, unlike developing Tregs in which the primary role of IL-2 is to initiate Foxp3 expression, mature Tregs require continuous IL-2 signaling to maintain survival and suppressor function, but not to maintain lineage stability.
Keywords: CD25; IL-15; IL-2; IL-7; regulatory T cell.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures

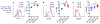

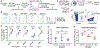
References
-
- Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, and Martin R (2006). Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl. Acad. Sci.USA 103, 5941–5946. - PMC - PubMed
-
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, and Farrar MA (2007). IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 178, 280–290. - PubMed
-
- Cornish GH, Sinclair LV, and Cantrell DA (2006). Differential regulation of T-cell growth by IL-2 and IL-15. Blood 108, 600–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

