iTRAQ-Based Quantitative Proteomic Analysis Reveals Changes in Metabolite Biosynthesis in Monascus purpureus in Response to a Low-Frequency Magnetic Field
- PMID: 30380661
- PMCID: PMC6267588
- DOI: 10.3390/toxins10110440
iTRAQ-Based Quantitative Proteomic Analysis Reveals Changes in Metabolite Biosynthesis in Monascus purpureus in Response to a Low-Frequency Magnetic Field
Abstract
Background: Low-frequency magnetic fields (LF-MFs) dampen the citrinin output by Monascus purpureus in fermentations. The influence of LF-MFs on biosynthesis by M. purpureus was evaluated at the protein level.
Methods: Cultures were treated with a 1.6-mT MF from day 0 to day 2 of incubation, and secondary metabolite production was evaluated on the day 12 of incubation. All proteins were extracted from M. purpureus mycelia and subjected to isobaric tags for relative and absolute quantification (iTRAQ) labeling and subsequent liquid chromatography/mass spectrometry (LC-MS/MS) analysis on day 6 of fermentation.
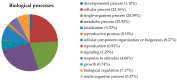
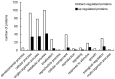
Results: There was no difference in biomass between the treated samples and the control. Citrinin production was 46.7% lower, and the yields of monacolin K and yellow, orange, and red pigment were 29.3%, 31.3%, 41.7%, and 40.3% higher, respectively, in the exposed samples compared to the control. Protein expression in M. purpureus under LF-MF treatment was quantified using iTRAQ technology. Of 2031 detected proteins, 205 were differentially expressed. The differentially-expressed proteins were subjected to Gene Ontology (GO) functional annotation and statistical analysis, which revealed that they mainly refer to biological metabolism, translation, antioxidant, transport and defense pathways. Among all the tagged proteins, emphasis was placed on the analysis of those involved in the synthesis of citrinin, pigment and monacolin K was emphasized.
Conclusions: LF-MFs affected Monascus secondary metabolism at the protein level, and aggregate data for all the protein profiles in LF-MF-treated Monascus was obtained.
Keywords: LF-MF; Monascus purpureus; citrinin; monacolin K; pigment; protein expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Diana M., Quílez J., Rafecas M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods. 2014;10:407–420. doi: 10.1016/j.jff.2014.07.004. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

