Liquid Biopsy in Gastrointestinal Cancers
- PMID: 30380690
- PMCID: PMC6316210
- DOI: 10.3390/diagnostics8040075
Liquid Biopsy in Gastrointestinal Cancers
Abstract
Liquid biopsy is the sampling of any biological fluid in an effort to enrich and analyze a tumor's genetic material. Peripheral blood remains the most studied liquid biopsy material, with circulating tumor cells (CTC's) and circulating tumor DNA (ctDNA) allowing the examination and longitudinal monitoring of a tumors genetic landscape. With applications in cancer screening, prognostic stratification, therapy selection and disease surveillance, liquid biopsy represents an exciting new paradigm in the field of cancer diagnostics and offers a less invasive and more comprehensive alternative to conventional tissue biopsy. Here, we examine liquid biopsies in gastrointestinal cancers, specifically colorectal, gastric, and pancreatic cancers, with an emphasis on applications in diagnostics, prognostics and therapeutics.
Keywords: circulating tumor DNA; circulating tumor cells; gastrointestinal cancer; liquid biopsy.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

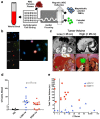
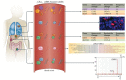

References
-
- Fitzmaurice C., Allen C., Barber R.M., Barregard L., Bhutta Z.A., Brenner H., Dicker D.J., Chimed-Orchir O., Dandona R., Dandona L., et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. - DOI - PMC - PubMed
-
- Allard W.J., Matera J., Miller M.C., Repollet M., Connelly M.C., Rao C., Tibbe A.G., Uhr J.W., Terstappen L.W. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but Not in Healthy Subjects or Patients with Nonmalignant Diseases. Clin. Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

