Mirtazapine added to SSRIs or SNRIs for treatment resistant depression in primary care: phase III randomised placebo controlled trial (MIR)
- PMID: 30381374
- PMCID: PMC6207929
- DOI: 10.1136/bmj.k4218
Mirtazapine added to SSRIs or SNRIs for treatment resistant depression in primary care: phase III randomised placebo controlled trial (MIR)
Erratum in
-
Mirtazapine added to SSRIs or SNRIs for treatment resistant depression in primary care: phase III randomised placebo controlled trial (MIR).BMJ. 2018 Nov 15;363:k4691. doi: 10.1136/bmj.k4691. BMJ. 2018. PMID: 30442772 No abstract available.
Abstract
Objective: To investigate the effectiveness of combining mirtazapine with serotonin-noradrenaline reuptake inhibitor (SNRI) or selective serotonin reuptake inhibitor (SSRI) antidepressants for treatment resistant depression in primary care.
Design: Two parallel group multicentre phase III randomised placebo controlled trial.
Setting: 106 general practices in four UK sites; Bristol, Exeter, Hull, and Keele/North Staffs, August 2013 to October 2015.
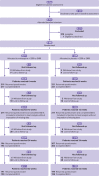
Participants: 480 adults aged 18 or more years who scored 14 or more on the Beck depression inventory, second revision, fulfilled ICD-10 (international classification of diseases, 10th revision) criteria for depression, and had used an SSRI or SNRI for at least six weeks but were still depressed. 241 were randomised to mirtazapine and 239 to placebo, both given in addition to usual SSRI or SNRI treatment. Participants were stratified by centre and minimised by baseline Beck depression inventory score, sex, and current psychological therapy. They were followed up at 12, 24, and 52 weeks. 431 (89.8%) were included in the (primary) 12 week follow-up.
Main outcome measures: Depressive symptoms at 12 weeks after randomisation, measured using the Beck depression inventory II score as a continuous variable. Secondary outcomes included measures of anxiety, quality of life, and adverse effects at 12, 24, and 52 weeks.
Results: Beck depression inventory II scores at 12 weeks were lower in the mirtazapine group after adjustment for baseline scores and minimisation or stratification variables, although the confidence interval included the null (mean (SD) scores at 12 weeks: 18.0 (12.3) in the mirtazapine group, 19.7 (12.4) in the placebo group; adjusted difference between means -1.83 (95% confidence interval -3.92 to 0.27); P=0.09). Adverse effects were more common in the mirtazapine group and were associated with the participants stopping the trial drug.
Conclusion: This study did not find evidence of a clinically important benefit for mirtazapine in addition to an SSRI or SNRI over placebo in a treatment resistant group of primary care patients with depression. This remains an area of important unmet need where evidence of effective treatment options is limited.
Trial registration: Current Controlled Trials ISRCTN06653773.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; DSK, JR, GL, NJW, and TJP report grants from NIHR HTA during the conduct of the study; and IMA reports personal fees from Lundbeck-Otsuka, Takeda, and Lundbeck outside the submitted work; there were no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Mirtazapin ist kein guter Therapiebooster.MMW Fortschr Med. 2018 Dec;160(21-22):38. doi: 10.1007/s15006-018-1228-z. MMW Fortschr Med. 2018. PMID: 30542867 German. No abstract available.
-
Adding mirtazapine to ongoing SNRIs or SSRIs did not improve symptoms of treatment-resistant depression.Ann Intern Med. 2019 Feb 19;170(4):JC20. doi: 10.7326/ACPJ201902190-020. Ann Intern Med. 2019. PMID: 30776809 No abstract available.
References
-
- Mathers C, Loncar D. Updated projections of global mortality and burden of disease, 2002-2030: data sources, methods and results (working paper). Geneva: WHO; 2005.
-
- Prescribing and Medicines Team, Health and Social Care Information Centre Prescriptions dispensed in the Community. 2015: 2004-14.
-
- National Collaborating Centre for Mental Health. The NICE Guideline on the Treatment and Management of Depression in Adults Updated Edition. National Institute for Health and Clinical Excellence, editor. London: The British Psychological Society and The Royal College of Psychiatrists; 2010.
-
- ICD-10 World Health Organization The ICD-10 classification of mental and behavioural disorders. 1992.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical