Sex Differences in Risk and Resilience: Stress Effects on the Neural Substrates of Emotion and Motivation
- PMID: 30381434
- PMCID: PMC6209838
- DOI: 10.1523/JNEUROSCI.1673-18.2018
Sex Differences in Risk and Resilience: Stress Effects on the Neural Substrates of Emotion and Motivation
Abstract
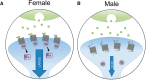
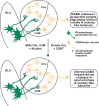
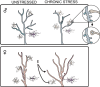
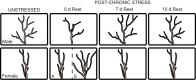
Risk for stress-sensitive psychopathologies differs in men and women, yet little is known about sex-dependent effects of stress on cellular structure and function in corticolimbic regions implicated in these disorders. Determining how stress influences these regions in males and females will deepen our understanding of the mechanisms underlying sex-biased psychopathology. Here, we discuss sex differences in CRF regulation of arousal and cognition, glucocorticoid modulation of amygdalar physiology and alcohol consumption, the age-dependent impact of social stress on prefrontal pyramidal cell excitability, stress effects on the prefrontal parvalbumin system in relation to emotional behaviors, contributions of stress and gonadal hormones to stress effects on prefrontal glia, and alterations in corticolimbic structure and function after cessation of chronic stress. These studies demonstrate that, while sex differences in stress effects may be nuanced, nonuniform, and nonlinear, investigations of these differences are nonetheless critical for developing effective, sex-specific treatments for psychological disorders.
Keywords: corticolimbic regions; corticosterone; corticotropin releasing factor; microglia; parvalbumin.
Copyright © 2018 the authors 0270-6474/18/389423-10$15.00/0.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
