p53 mutants cooperate with HIF-1 in transcriptional regulation of extracellular matrix components to promote tumor progression
- PMID: 30381462
- PMCID: PMC6243248
- DOI: 10.1073/pnas.1808314115
p53 mutants cooperate with HIF-1 in transcriptional regulation of extracellular matrix components to promote tumor progression
Abstract
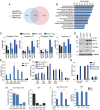
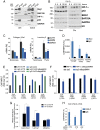
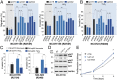
Mutations in the TP53 gene and microenvironmentally driven activation of hypoxia-inducible factor-1 (HIF-1) typically occur in later stages of tumorigenesis. An ongoing challenge is the identification of molecular determinants of advanced cancer pathogenesis to design alternative last-line therapeutic options. Here, we report that p53 mutants influence the tumor microenvironment by cooperating with HIF-1 to promote cancer progression. We demonstrate that in non-small cell lung cancer (NSCLC), p53 mutants exert a gain-of-function (GOF) effect on HIF-1, thus regulating a selective gene expression signature involved in protumorigenic functions. Hypoxia-mediated activation of HIF-1 leads to the formation of a p53 mutant/HIF-1 complex that physically binds the SWI/SNF chromatin remodeling complex, promoting expression of a selective subset of hypoxia-responsive genes. Depletion of p53 mutants impairs the HIF-mediated up-regulation of extracellular matrix (ECM) components, including type VIIa1 collagen and laminin-γ2, thus affecting tumorigenic potential of NSCLC cells in vitro and in mouse models in vivo. Analysis of surgically resected human NSCLC revealed that expression of this ECM gene signature was highly correlated with hypoxic tumors exclusively in patients carrying p53 mutations and was associated with poor prognosis. Our data reveal a GOF effect of p53 mutants in hypoxic tumors and suggest synergistic activities of p53 and HIF-1. These findings have important implications for cancer progression and might provide innovative last-line treatment options for advanced NSCLC.
Keywords: HIF; SWI/SNF; chromatin architecture; microenvironment; p53.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

