International consensus on quality standards for brain health-focused care in multiple sclerosis
- PMID: 30381987
- PMCID: PMC6826858
- DOI: 10.1177/1352458518809326
International consensus on quality standards for brain health-focused care in multiple sclerosis
Abstract
Background: Time matters in multiple sclerosis (MS). Irreversible neural damage and cell loss occur from disease onset. The MS community has endorsed a management strategy of prompt diagnosis, timely intervention and regular proactive monitoring of treatment effectiveness and disease activity to improve outcomes in people with MS.
Objectives: We sought to develop internationally applicable quality standards for timely, brain health-focused MS care.
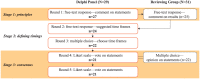
Methods: A panel of MS specialist neurologists participated in an iterative, online, modified Delphi process to define 'core', 'achievable' and 'aspirational' time frames reflecting minimum, good and high care standards, respectively. A multidisciplinary Reviewing Group (MS nurses, people with MS, allied healthcare professionals) provided insights ensuring recommendations reflected perspectives from multiple stakeholders.
Results: Twenty-one MS neurologists from 19 countries reached consensus on most core (25/27), achievable (25/27) and aspirational (22/27) time frames at the end of five rounds. Agreed standards cover six aspects of the care pathway: symptom onset, referral and diagnosis, treatment decisions, lifestyle, disease monitoring and managing new symptoms.
Conclusion: These quality standards for core, achievable and aspirational care provide MS teams with a three-level framework for service evaluation, benchmarking and improvement. They have the potential to produce a profound change in the care of people with MS.
Keywords: Delphi technique; Multiple sclerosis; benchmarking; consensus; quality improvement; standards.
Conflict of interest statement
Figures


Comment in
-
Cognition and its relation to brain health in patients with MS: Response to letter.Mult Scler. 2020 Oct;26(12):1613-1614. doi: 10.1177/1352458520918376. Epub 2020 May 6. Mult Scler. 2020. PMID: 32372708 Free PMC article. No abstract available.
-
Cognition and its relation to brain health in patients with MS.Mult Scler. 2020 Oct;26(12):1611-1613. doi: 10.1177/1352458520907906. Epub 2020 May 6. Mult Scler. 2020. PMID: 32372717 Free PMC article. No abstract available.
References
-
- Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 2008; 79: 1368–1374. - PubMed
-
- Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: Time matters in multiple sclerosis. Mult Scler Relat Disord 2016; 9(Suppl. 1): S5–S48. - PubMed
-
- Kappos L, Traboulsee A, Constantinescu C, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology 2006; 67: 944–953. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

