Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics
- PMID: 30382084
- PMCID: PMC6208419
- DOI: 10.1038/s41467-018-06730-z
Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics
Abstract
The progress of nanoparticle (NP)-based drug delivery has been hindered by an inability to establish structure-activity relationships in vivo. Here, using stable, monosized, radiolabeled, mesoporous silica nanoparticles (MSNs), we apply an integrated SPECT/CT imaging and mathematical modeling approach to understand the combined effects of MSN size, surface chemistry and routes of administration on biodistribution and clearance kinetics in healthy rats. We show that increased particle size from ~32- to ~142-nm results in a monotonic decrease in systemic bioavailability, irrespective of route of administration, with corresponding accumulation in liver and spleen. Cationic MSNs with surface exposed amines (PEI) have reduced circulation, compared to MSNs of identical size and charge but with shielded amines (QA), due to rapid sequestration into liver and spleen. However, QA show greater total excretion than PEI and their size-matched neutral counterparts (TMS). Overall, we provide important predictive functional correlations to support the rational design of nanomedicines.
Conflict of interest statement
The authors declare no competing interests.
Figures


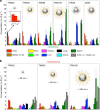

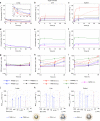

References
-
- Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. doi: 10.1038/natrevmats.2016.14. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- DTRA100279003/DOD | Defense Threat Reduction Agency (DTRA)/International
- R01 CA222007/CA/NCI NIH HHS/United States
- U01 CA213759/CA/NCI NIH HHS/United States
- U54 CA210181/CA/NCI NIH HHS/United States
- DMS-1716737/National Science Foundation (NSF)/International
- P50GM085273/U.S. Department of Health & Human Services | National Institutes of Health (NIH)/International
- DE-NA0003525/U.S. Department of Energy (DOE)/International
- U54CA210181/University of Texas System (UT)/International
- DTRA1002713506/DOD | Defense Threat Reduction Agency (DTRA)/International
- U01 CA196403/CA/NCI NIH HHS/United States
- P50 GM085273/GM/NIGMS NIH HHS/United States
- 1U01CA196403/U.S. Department of Health & Human Services | National Institutes of Health (NIH)/International
- R01 CA226537/CA/NCI NIH HHS/United States
- R01 MH096093/MH/NIMH NIH HHS/United States
- 1U01CA213759/U.S. Department of Health & Human Services | National Institutes of Health (NIH)/International
- DMS-1562068/National Science Foundation (NSF)/International
- 1R01CA226537/U.S. Department of Health & Human Services | National Institutes of Health (NIH)/International
- 1R01CA222007/U.S. Department of Health & Human Services | National Institutes of Health (NIH)/International
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

