Lévy-like movement patterns of metastatic cancer cells revealed in microfabricated systems and implicated in vivo
- PMID: 30382086
- PMCID: PMC6208440
- DOI: 10.1038/s41467-018-06563-w
Lévy-like movement patterns of metastatic cancer cells revealed in microfabricated systems and implicated in vivo
Abstract
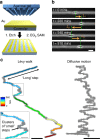
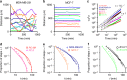
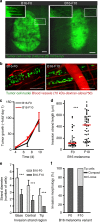
Metastatic cancer cells differ from their non-metastatic counterparts not only in terms of molecular composition and genetics, but also by the very strategy they employ for locomotion. Here, we analyzed large-scale statistics for cells migrating on linear microtracks to show that metastatic cancer cells follow a qualitatively different movement strategy than their non-invasive counterparts. The trajectories of metastatic cells display clusters of small steps that are interspersed with long "flights". Such movements are characterized by heavy-tailed, truncated power law distributions of persistence times and are consistent with the Lévy walks that are also often employed by animal predators searching for scarce prey or food sources. In contrast, non-metastatic cancerous cells perform simple diffusive movements. These findings are supported by preliminary experiments with cancer cells migrating away from primary tumors in vivo. The use of chemical inhibitors targeting actin-binding proteins allows for "reprogramming" the Lévy walks into either diffusive or ballistic movements.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Stokes CL, Lauffenburger DA, Williams SK. Migration of individual microvessel endothelial cells: stochastic model and parameter measurement. J. Cell. Sci. 1991;99:419–430. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

