Interactions between callose and cellulose revealed through the analysis of biopolymer mixtures
- PMID: 30382102
- PMCID: PMC6208431
- DOI: 10.1038/s41467-018-06820-y
Interactions between callose and cellulose revealed through the analysis of biopolymer mixtures
Abstract
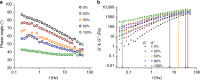
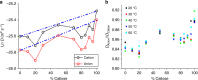
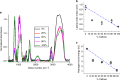
The properties of (1,3)-β-glucans (i.e., callose) remain largely unknown despite their importance in plant development and defence. Here we use mixtures of (1,3)-β-glucan and cellulose, in ionic liquid solution and hydrogels, as proxies to understand the physico-mechanical properties of callose. We show that after callose addition the stiffness of cellulose hydrogels is reduced at a greater extent than predicted from the ideal mixing rule (i.e., the weighted average of the individual components' properties). In contrast, yield behaviour after the elastic limit is more ductile in cellulose-callose hydrogels compared with sudden failure in 100% cellulose hydrogels. The viscoelastic behaviour and the diffusion of the ions in mixed ionic liquid solutions strongly indicate interactions between the polymers. Fourier-transform infrared analysis suggests that these interactions impact cellulose organisation in hydrogels and cell walls. We conclude that polymer interactions alter the properties of callose-cellulose mixtures beyond what it is expected by ideal mixing.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Bacic, A., Fincher, G. B., Stone, B. A. Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides, First edn. (Academic press, Elsevier, USA, 2009).
-
- Carreño Neftali L. V., Barbosa Ananda M., Noremberg Bruno S., Salas Mabel M. S., Fernandes Susana C. M., Labidi Jalel. Advances in Nanostructured Cellulose-based Biomaterials. Cham: Springer International Publishing; 2017. Advances in Nanostructured Cellulose-based Biomaterials; pp. 1–32.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

