Metabolism and disposition of pyrotinib in healthy male volunteers: covalent binding with human plasma protein
- PMID: 30382184
- PMCID: PMC6786348
- DOI: 10.1038/s41401-018-0176-6
Metabolism and disposition of pyrotinib in healthy male volunteers: covalent binding with human plasma protein
Abstract
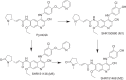
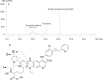
Pyrotinib is a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor that is used to treat HER2-positive breast cancer. In this study we investigated the metabolism and disposition of pyrotinib in six healthy Chinese men after a single oral dose of 402 mg of [14C]pyrotinib. At 240 h postdose, the mean cumulative excretion of the dose radioactivity was 92.6%, including 1.7% in urine and 90.9% in feces. In feces, oxidative metabolites were detected as major drug-related materials and the primary metabolic pathways were O-depicoline (M1), oxidation of pyrrolidine (M5), and oxidation of pyridine (M6-1, M6-2, M6-3, and M6-4). In plasma, the major circulating entities identified were pyrotinib, SHR150980 (M1), SHR151468 (M2), and SHR151136 (M5), accounting for 10.9%, 1.9%, 1.0%, and 3.0%, respectively, of the total plasma radioactivity based on the AUC0-∞ ratios. Approximately 58.3% of the total plasma radioactivity AUC0-∞ was attributed to covalently bound materials. After incubation of human plasma with [14C]pyrotinib at 37 °C for 2, 5, 8, and 24 h, the recovery of radioactivity by extraction was 97.4%, 91.8%, 69.6%, and 46.7%, respectively, revealing covalent binding occurred independently of enzymes. A group of pyrotinib adducts, including pyrotinib-lysine and pyrotinib adducts of the peptides Gly-Lys, Lys-Ala, Gly-Lys-Ala, and Lys-Ala-Ser, was identified after HCl hydrolysis of the incubated plasma. Therefore, the amino acid residue Lys190 of human serum albumin was proposed to covalently bind to pyrotinib via Michael addition. Finally, the covalently bound pyrotinib could dissociate from the human plasma protein and be metabolized by oxidation and excreted via feces.
Keywords: EGFR/HER2 dual tyrosine kinase inhibitor; breast cancer; covalent binding; drug disposition; drug metabolism; human plasma; pyrotinib.
Conflict of interest statement
The authors declare that there is no competing interests.
Figures



References
-
- Li X, Yang CY, Wan H, Zhang G, Feng J, Zhang L, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51–61. doi: 10.1016/j.ejps.2017.01.021. - DOI - PubMed
-
- Ma F, Li Q, Chen SS, Zhu WJ, Fan Y, Wang JY, et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible Pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2017;35:3105–12. doi: 10.1200/JCO.2016.69.6179. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

