Corticohippocampal Dysfunction In The OBiden Mouse Model Of Primary Oligodendrogliopathy
- PMID: 30382234
- PMCID: PMC6208344
- DOI: 10.1038/s41598-018-34414-7
Corticohippocampal Dysfunction In The OBiden Mouse Model Of Primary Oligodendrogliopathy
Abstract
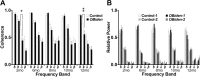
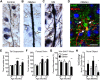
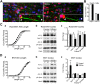
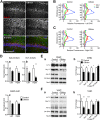
Despite concerted efforts over decades, the etiology of multiple sclerosis (MS) remains unclear. Autoimmunity, environmental-challenges, molecular mimicry and viral hypotheses have proven equivocal because early-stage disease is typically presymptomatic. Indeed, most animal models of MS also lack defined etiologies. We have developed a novel adult-onset oligodendrogliopathy using a delineated metabolic stress etiology in myelinating cells, and our central question is, "how much of the pathobiology of MS can be recapitulated in this model?" The analyses described herein demonstrate that innate immune activation, glial scarring, cortical and hippocampal damage with accompanying electrophysiological, behavioral and memory deficits naturally emerge from disease progression. Molecular analyses reveal neurofilament changes in normal-appearing gray matter that parallel those in cortical samples from MS patients with progressive disease. Finally, axon initial segments of deep layer pyramidal neurons are perturbed in entorhinal/frontal cortex and hippocampus from OBiden mice, and computational modeling provides insight into vulnerabilities of action potential generation during demyelination and early remyelination. We integrate these findings into a working model of corticohippocampal circuit dysfunction to predict how myelin damage might eventually lead to cognitive decline.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- MH107512/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)/International
- NS021135/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)/International
- NS043783/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)/International
- NS067157/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)/International
- RG2891/National Multiple Sclerosis Society (National MS Society)/International
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

