Primary Adrenal Insufficiency During Lenvatinib or Vandetanib and Improvement of Fatigue After Cortisone Acetate Therapy
- PMID: 30383218
- PMCID: PMC6402317
- DOI: 10.1210/jc.2018-01836
Primary Adrenal Insufficiency During Lenvatinib or Vandetanib and Improvement of Fatigue After Cortisone Acetate Therapy
Abstract
Context: Two tyrosine kinase inhibitors (TKIs), lenvatinib and vandetanib, are often used to treat advanced radioiodine-refractory differentiated thyroid cancer (RAI-R DTC) and medullary thyroid cancer (MTC), respectively. Fatigue is a common adverse event during treatment with these and other TKIs and a common cause of drug discontinuation or dosage reduction.
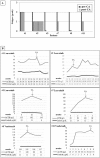
Cases description: We evaluated the basal and stimulated adrenal function in 12 patients with advanced RAI-R DTC and MTC treated with lenvatinib or vandetanib, respectively. Ten patients complaining of fatigue showed a progressive ACTH increase with normal cortisol levels. Moreover, six of 10 patients had a blunted cortisol response after ACTH stimulation, thus confirming the diagnosis of primary adrenal insufficiency (PAI). The causal relationship between TKIs and PAI onset was also demonstrated by the repeated testing of adrenal function before and during treatment. Patients with PAI received cortisone acetate replacement therapy, with a substantial and prompt improvement in the degree of fatigue, as assessed by the Common Terminology Criteria for Adverse Events version 4.03, thus supporting the major impact of impaired adrenal function in the genesis of this adverse event.
Conclusions: We show that the occurrence of PAI may be a common cause of fatigue during lenvatinib and vandetanib treatment, and we therefore recommend testing adrenal function for a prompt start of replacement therapy to avoid treatment discontinuation, dosage reduction, and potentially severe PAI complications.
Figures

References
-
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. - PubMed
-
- Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. - PMC - PubMed
-
- Brose MS, Bible KC, Chow LQM, Gilbert J, Grande C, Worden F, Haddad R. Management of treatment-related toxicities in advanced medullary thyroid cancer. Cancer Treat Rev. 2018;66:64–73. - PubMed
-
- Bilgir O, Kebapcilar L, Bilgir F, Sarì I, Oner P, Karaca B, Alacacioglu I. Is there any relationship between imatinib mesylate medication and hypothalamic-pituitary-adrenal axis dysfunction? Int J Clin Pract. 2010;64(1):45–50. - PubMed
-
- Brassard M, Neraud B, Trabado S, Salenave S, Brailly-Tabard S, Borget I, Baudin E, Leboulleux S, Chanson P, Schlumberger M, Young J. Endocrine effects of the tyrosine kinase inhibitor vandetanib in patients treated for thyroid cancer. J Clin Endocrinol Metab. 2011;96(9):2741–2749. - PubMed

