Characterization of the Genital Mucosa Immune Profile to Distinguish Phases of the Menstrual Cycle: Implications for HIV Susceptibility
- PMID: 30383238
- PMCID: PMC6386813
- DOI: 10.1093/infdis/jiy585
Characterization of the Genital Mucosa Immune Profile to Distinguish Phases of the Menstrual Cycle: Implications for HIV Susceptibility
Abstract
Background: Inflammation and immune activation are key factors in sexual transmission of human immunodeficiency virus (HIV). We sought to define the impact of hormonal cycling on the mucosal immune environment and HIV risk in sex workers with a natural menstrual cycle.
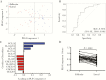
Methods: We compared soluble mucosal immune factors and cervical mononuclear cells during hormone titer-defined phases of the menstrual cycle among 37 sex workers from Nairobi, Kenya. Systemic and mucosal samples were collected 14 days apart to distinguish the follicular and luteal phases of the menstrual cycle, and phases were confirmed by hormone measurements. Vaginal concentrations of 19 immune modulators and cervical T-cell activation markers were measured.
Results: The follicular phase signature was characterized by an elevated CCL2 level, decreased interleukin 1α and interleukin 1β cervical concentrations, and a significant increase in the proportion of CD4+ T cells that expressed CD69. The genital concentration of CCL2 was the best marker to distinguish the follicular from the luteal phase in univariate and multivariate analyses and remained independent of elevated genital inflammation and bacterial vaginosis.
Conclusion: The follicular phase of the menstrual cycle was associated with an elevated CCL2 level and retention of resident memory CD4+ T cells, which has implications for increased susceptibility to HIV infection.
Keywords: Genital inflammation; HIV; immune activation; menstrual cycle.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- Joint United Nations Programme on HIV. UNAIDS data 2017. Geneva: UNAIDS, 2017:1–246.
-
- Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med 2011; 62:127–39. - PubMed
-
- Lajoie J, Juno J, Burgener A, et al. . A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol 2012; 5:277–87. - PubMed

