Development of a Rhinovirus Inoculum Using a Reverse Genetics Approach
- PMID: 30383246
- PMCID: PMC6581892
- DOI: 10.1093/infdis/jiy629
Development of a Rhinovirus Inoculum Using a Reverse Genetics Approach
Abstract
Background: Experimental inoculation is an important tool for common cold and asthma research. Producing rhinovirus (RV) inocula from nasal secretions has required prolonged observation of the virus donor to exclude extraneous pathogens. We produced a RV-A16 inoculum using reverse genetics and determined the dose necessary to cause moderate colds in seronegative volunteers.
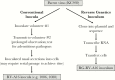
Methods: The consensus sequence of RV-A16 from a previous inoculum was cloned, and inoculum virus was produced using reverse genetics techniques. After safety testing, volunteers were inoculated with either RV-A16 (n = 26) or placebo (n = 10), Jackson cold scores were recorded, and nasal secretions were tested for shedding of RV-A16 ribonucleic acid.
Results: The reverse genetics process produced infectious virus that was neutralized by specific antisera and had a mutation rate similar to conventional virus growth techniques. The 1000 median tissue culture infectious dose (TCID50) dose produced moderate colds in most individuals with effects similar to that of a previously tested conventional RV-A16 inoculum.
Conclusions: Reverse genetics techniques produced a RV-A16 inoculum that can cause clinical colds in seronegative volunteers, and they also serve as a stable source of virus for laboratory use. The recombinant production procedures eliminate the need to derive seed virus from nasal secretions, thus precluding introduction of extraneous pathogens through this route.
Keywords: common cold; inoculation; reverse genetics; rhinovirus.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures



Comment in
-
Reverse Genetics and Rhinovirus-A New Approach to an Old Problem?J Infect Dis. 2019 Jun 19;220(2):181-183. doi: 10.1093/infdis/jiy630. J Infect Dis. 2019. PMID: 30383231 Free PMC article. No abstract available.
Similar articles
-
Analysis of tracheal secretions for rhinovirus during natural colds.Pediatr Allergy Immunol. 2005 May;16(3):276-8. doi: 10.1111/j.1399-3038.2005.00246.x. Pediatr Allergy Immunol. 2005. PMID: 15853960
-
Experimental rhinovirus infection in volunteers.Eur Respir J. 1996 Nov;9(11):2250-5. doi: 10.1183/09031936.96.09112250. Eur Respir J. 1996. PMID: 8947068
-
Epitope mapping of antibodies induced with a conserved rhinovirus protein generating protective anti-rhinovirus immunity.Vaccine. 2019 May 9;37(21):2805-2813. doi: 10.1016/j.vaccine.2019.04.018. Epub 2019 Apr 16. Vaccine. 2019. PMID: 31003914
-
Effects on the nasal mucosa of upper respiratory viruses (common cold).Dan Med Bull. 1994 Apr;41(2):193-204. Dan Med Bull. 1994. PMID: 8039434 Review.
-
[Diagnostic tests: Rhinovirus].Nihon Rinsho. 2005 Jul;63 Suppl 7:366-9. Nihon Rinsho. 2005. PMID: 16111276 Review. Japanese. No abstract available.
Cited by
-
Construction and characterization of an infectious cDNA clone of human rhinovirus A89.Heliyon. 2024 Feb 28;10(5):e27214. doi: 10.1016/j.heliyon.2024.e27214. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38463855 Free PMC article.
-
Assessing immune factors in maternal milk and paired infant plasma antibody binding to human rhinoviruses.Front Immunol. 2024 Jul 25;15:1385121. doi: 10.3389/fimmu.2024.1385121. eCollection 2024. Front Immunol. 2024. PMID: 39119337 Free PMC article.
-
Risk analysis for outpatient experimental infection as a pathway for affordable RSV vaccine development.NPJ Vaccines. 2025 Apr 15;10(1):70. doi: 10.1038/s41541-025-01125-w. NPJ Vaccines. 2025. PMID: 40234435 Free PMC article.
-
Reverse Genetics and Rhinovirus-A New Approach to an Old Problem?J Infect Dis. 2019 Jun 19;220(2):181-183. doi: 10.1093/infdis/jiy630. J Infect Dis. 2019. PMID: 30383231 Free PMC article. No abstract available.
-
Epithelial cell responses to rhinovirus identify an early-life-onset asthma phenotype in adults.J Allergy Clin Immunol. 2022 Sep;150(3):604-611. doi: 10.1016/j.jaci.2022.03.020. Epub 2022 Mar 31. J Allergy Clin Immunol. 2022. PMID: 35367470 Free PMC article.
References
-
- Gern JE, Palmenberg AC. Rhinoviruses. In: Knipe DM, Howley PM, eds. Field’s Virology. Philadelphia: Lippincott Williams & Wilkins, 2013.
-
- D’Alessio DJ, Meschievitz CK, Peterson JA, Dick CR, Dick EC. Short-duration exposure and the transmission of rhinoviral colds. J Infect Dis 1984; 150:189–94. - PubMed
-
- Turner BW, Cail WS, Hendley JO, et al. . Physiologic abnormalities in the paranasal sinuses during experimental rhinovirus colds. J Allergy Clin Immunol 1992; 90:474–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

