A planarian nidovirus expands the limits of RNA genome size
- PMID: 30383829
- PMCID: PMC6211748
- DOI: 10.1371/journal.ppat.1007314
A planarian nidovirus expands the limits of RNA genome size
Abstract
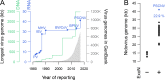
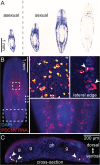
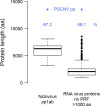
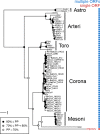
RNA viruses are the only known RNA-protein (RNP) entities capable of autonomous replication (albeit within a permissive host environment). A 33.5 kilobase (kb) nidovirus has been considered close to the upper size limit for such entities; conversely, the minimal cellular DNA genome is in the 100-300 kb range. This large difference presents a daunting gap for the transition from primordial RNP to contemporary DNA-RNP-based life. Whether or not RNA viruses represent transitional steps towards DNA-based life, studies of larger RNA viruses advance our understanding of the size constraints on RNP entities and the role of genome size in virus adaptation. For example, emergence of the largest previously known RNA genomes (20-34 kb in positive-stranded nidoviruses, including coronaviruses) is associated with the acquisition of a proofreading exoribonuclease (ExoN) encoded in the open reading frame 1b (ORF1b) in a monophyletic subset of nidoviruses. However, apparent constraints on the size of ORF1b, which encodes this and other key replicative enzymes, have been hypothesized to limit further expansion of these viral RNA genomes. Here, we characterize a novel nidovirus (planarian secretory cell nidovirus; PSCNV) whose disproportionately large ORF1b-like region including unannotated domains, and overall 41.1-kb genome, substantially extend the presumed limits on RNA genome size. This genome encodes a predicted 13,556-aa polyprotein in an unconventional single ORF, yet retains canonical nidoviral genome organization and expression, as well as key replicative domains. These domains may include functionally relevant substitutions rarely or never before observed in highly conserved sites of RdRp, NiRAN, ExoN and 3CLpro. Our evolutionary analysis suggests that PSCNV diverged early from multi-ORF nidoviruses, and acquired additional genes, including those typical of large DNA viruses or hosts, e.g. Ankyrin and Fibronectin type II, which might modulate virus-host interactions. PSCNV's greatly expanded genome, proteomic complexity, and unique features-impressive in themselves-attest to the likelihood of still-larger RNA genomes awaiting discovery.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

