Pathology of experimentally induced mouthrot caused by Tenacibaculum maritimum in Atlantic salmon smolts
- PMID: 30383870
- PMCID: PMC6211739
- DOI: 10.1371/journal.pone.0206951
Pathology of experimentally induced mouthrot caused by Tenacibaculum maritimum in Atlantic salmon smolts
Abstract
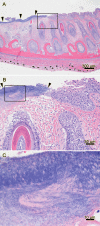
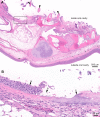
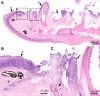
Mouthrot, caused by Tenacibaculum maritimum is a significant disease of farmed Atlantic salmon, Salmo salar on the West Coast of North America. Smolts recently transferred into saltwater are the most susceptible and affected fish die with little internal or external clinical signs other than the characteristic small (usually < 5 mm) yellow plaques that are present inside the mouth. The mechanism by which these smolts die is unknown. This study investigated the microscopic pathology (histology and scanning electron microscopy) of bath infected smolts with Western Canadian T. maritimum isolates TmarCan15-1, TmarCan16-1 and TmarCan16-5 and compared the findings to what is seen in a natural outbreak of mouthrot. A real-time RT-PCR assay based on the outer membrane protein A specific for T. maritimum was designed and used to investigate the tissue tropism of the bacteria. The results from this showed that T. maritimum is detectable internally by real-time RT-PCR. This combined with the fact that the bacteria can be isolated from the kidney suggests that T. maritimum becomes systemic. The pathology in the infected smolts is primarily mouth lesions, including damaged tissues surrounding the teeth; the disease is similar to periodontal disease in mammals. The pathological changes are focal, severe, and occur very rapidly with little associated inflammation. Skin lesions are more common in experimentally infected smolts than in natural outbreaks, but this could be an artefact of the challenge dose, handling and tank used during the experiments.
Conflict of interest statement
We have the following interests: Kathleen Frisch, Sverre Bang Småge, Henrik Duesund and Øyvind Jakobsen Brevik are employed by Cermaq Group AS, who planned and performed this study, and Renate Johansen is employed by Pharmaq Analytiq, who helped analyse the data. There are no patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures






References
-
- Toranzo AE, Magariños B, Romalde JL. A review of the main bacterial fish diseases in mariculture systems. Aquaculture. 2005;246: 37–61. 10.1016/j.aquaculture.2005.01.002 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

