The burden of invasive infections in neutropenic patients: incidence, outcomes, and use of granulocyte transfusions
- PMID: 30383912
- PMCID: PMC7379528
- DOI: 10.1111/trf.14994
The burden of invasive infections in neutropenic patients: incidence, outcomes, and use of granulocyte transfusions
Abstract
Background: Patients with prolonged neutropenia caused by chemotherapy or underlying marrow disorders are at risk of invasive bacterial and fungal infections. New treatment options alongside targeted antimicrobial therapy that might improve outcomes include granulocyte transfusions (GTX). To inform the research agenda, a prospective observational cohort study was performed in the Netherlands and United Kingdom. The aim was to describe the incidence, characteristics, and outcomes of patients developing invasive infections and assess patients fulfilling criteria for GTX.
Study design and methods: All patients receiving myeloablative chemotherapy and anticipated to develop 7 or more days of neutropenia (<0.5 × 109 /L) were eligible and followed for the development of invasive infections according to a defined algorithm and mortality up to 100 days. Secondary outcomes were types of infection and eligibility for GTX.
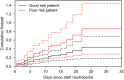
Results: A total of 471 patients enrolled at six hematology-oncology departments were followed for 569 neutropenic episodes. Overall, 32.5% of patients developed invasive infections during their first episode. Significant baseline risk factors for developing infections were high comorbidity scores (WHO performance status ≥ 2, hazard ratio [HR], 2.6 [1.7-3.9]; and hematopoietic cell transplantation-comorbidity index score ≥ 2 HR 1.3 [0.9-1.8]). Infections were bacterial (59.4%) and fungal (22.3%). Despite 34 patients (6.3% of all episodes) appearing to meet criteria to receive GTX, only nine patients received granulocytes. The HR for death was 5.8 (2.5-13.0) for patients with invasive infections.
Conclusion: This study documents that invasive infections are associated with significant mortality. There is a need for new strategies to prevent and treat infections, which may include better understanding of use GTX.
© 2018 The Authors. Transfusion published by Wiley Periodicals, Inc. on behalf of AABB.
Conflict of interest statement
The authors have disclosed no conflicts of interest.
Figures

Comment in
-
Therapeutic granulocyte transfusions: neutropenic patients with acute leukemia continue to need them - why are definitive evidence-based practice guidelines elusive?Transfusion. 2019 Jan;59(1):6-8. doi: 10.1111/trf.15025. Transfusion. 2019. PMID: 30615809 No abstract available.
References
-
- Lyman GH, Michels SL, Reynolds MW, et al. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 2010;116:5555–63. - PubMed
-
- Pfaller M, Neofytos D, Diekema D, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the prospective antifungal therapy (PATH Alliance(R)) registry, 2004‐2008. Diagn Microbiol Infect Dis 2012;74:323–31. - PubMed
-
- Arendrup MC. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect 2014;20(Suppl 6):42–8. - PubMed
-
- Stanworth SJ, Massey E, Hyde C, et al. Granulocyte transfusions for treating infections in patients with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev 2005;3:Cd005339. - PubMed
-
- Seidel MG, Peters C, Wacker A, et al. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant 2008;42:679–84. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

