New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis
- PMID: 30384259
- PMCID: PMC6205410
- DOI: 10.1016/j.redox.2018.09.025
New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis
Abstract
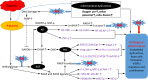
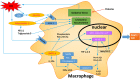
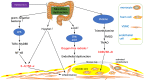
Oxidative stress and inflammation interact in the development of diabetic atherosclerosis. Intracellular hyperglycemia promotes production of mitochondrial reactive oxygen species (ROS), increased formation of intracellular advanced glycation end-products, activation of protein kinase C, and increased polyol pathway flux. ROS directly increase the expression of inflammatory and adhesion factors, formation of oxidized-low density lipoprotein, and insulin resistance. They activate the ubiquitin pathway, inhibit the activation of AMP-protein kinase and adiponectin, decrease endothelial nitric oxide synthase activity, all of which accelerate atherosclerosis. Changes in the composition of the gut microbiota and changes in microRNA expression that influence the regulation of target genes that occur in diabetes interact with increased ROS and inflammation to promote atherosclerosis. This review highlights the consequences of the sustained increase of ROS production and inflammation that influence the acceleration of atherosclerosis by diabetes. The potential contributions of changes in the gut microbiota and microRNA expression are discussed.
Keywords: Atherosclerosis; Diabetes mellitus; Gut microbiota; MicroRNA; Reactive oxygen species.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. - PubMed
-
- Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013;93:137–188. - PubMed
-
- Nouwen A., Nefs G., Caramlau I., Connock M., Winkley K., Lloyd C.E., Peyrot M., Pouwer F. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care. 2011;34:752–762. - PMC - PubMed
-
- Ross S., Gerstein H., Paré G. The genetic link between diabetes and atherosclerosis. Can. J. Cardiol. 2018;34:565–574. - PubMed
-
- Russell R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

