Prosaposin promotes the proliferation and tumorigenesis of glioma through toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway
- PMID: 30385233
- PMCID: PMC6286187
- DOI: 10.1016/j.ebiom.2018.10.053
Prosaposin promotes the proliferation and tumorigenesis of glioma through toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway
Abstract
Background: As a neurotrophic factor, prosaposin (PSAP) can exert neuroprotective and neurotrophic effects. It is involved in the occurrence and development of prostate and breast cancer. However, there is no research about the role of PSAP in glioma.
Methods: The PSAP overexpressed or silenced glioma cells or glioma stem cells were established based on Lentiviral vector transfection. Cell viability assay, Edu assay, neurosphere formation assay and xenograft experiments were used to detect the proliferative ability. Western blot, Elisa and luciferase reporter assays were used to detect the possible mechanism.
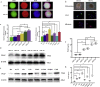
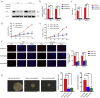
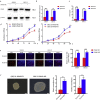
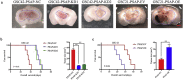
Findings: Our study firstly found that PSAP was highly expressed and secreted in clinical glioma specimens, glioma stem cells, and glioma cell lines. It was associated with poor prognosis. We found that PSAP significantly promoted the proliferation of glioma stem cells and cell lines. Moreover, PSAP promoted tumorigenesis in subcutaneous and orthotopic models of this disease. Furthermore, GSEA and KEGG analysis predicted that PSAP acts through the TLR4 and NF-κB signaling pathways, which was confirmed by western blot, immunoprecipitation, immunofluorescence, and use of the TLR4-specific inhibitor TAK-242.
Interpretation: The findings of this study suggest that PSAP can promote glioma cell proliferation via the TLR4/NF-κB signaling pathway and may be an important target for glioma treatment. FUND: This work was funded by National Natural Science Foundation of China (Nos. 81101917, 81270036, 81201802, 81673025), Program for Liaoning Excellent Talents in University (No. LR2014023), and Liaoning Province Natural Science Foundation (Nos. 20170541022, 20172250290). The funders did not play a role in manuscript design, data collection, data analysis, interpretation nor writing of the manuscript.
Keywords: Glioma; Glioma stem cells; Proliferation; Prosaposin; Tumorigenesis.
Copyright © 2018. Published by Elsevier B.V.
Figures





Similar articles
-
MiR-18a-downregulated RORA inhibits the proliferation and tumorigenesis of glioma using the TNF-α-mediated NF-κB signaling pathway.EBioMedicine. 2020 Feb;52:102651. doi: 10.1016/j.ebiom.2020.102651. Epub 2020 Feb 12. EBioMedicine. 2020. PMID: 32062354 Free PMC article.
-
Prosaposin is a biomarker of mesenchymal glioblastoma and regulates mesenchymal transition through the TGF-β1/Smad signaling pathway.J Pathol. 2019 Sep;249(1):26-38. doi: 10.1002/path.5278. Epub 2019 May 21. J Pathol. 2019. PMID: 30953361
-
Cullin-7 (CUL7) is overexpressed in glioma cells and promotes tumorigenesis via NF-κB activation.J Exp Clin Cancer Res. 2020 Apr 6;39(1):59. doi: 10.1186/s13046-020-01553-7. J Exp Clin Cancer Res. 2020. PMID: 32252802 Free PMC article.
-
RACK1 promotes NF-κB pathway activation and glioma cell proliferation by inhibiting RPS2 ubiquitination.Mol Biol Rep. 2025 Jun 16;52(1):600. doi: 10.1007/s11033-025-10691-0. Mol Biol Rep. 2025. PMID: 40522528
-
Dissecting the roles of prosaposin as an emerging therapeutic target for tumors and its underlying mechanisms.Biomed Pharmacother. 2024 Nov;180:117551. doi: 10.1016/j.biopha.2024.117551. Epub 2024 Oct 13. Biomed Pharmacother. 2024. PMID: 39405903 Review.
Cited by
-
Dysregulation of inflammasome activation in glioma.Cell Commun Signal. 2023 Sep 18;21(1):239. doi: 10.1186/s12964-023-01255-5. Cell Commun Signal. 2023. PMID: 37723542 Free PMC article. Review.
-
Glioblastoma biomarkers in urinary extracellular vesicles reveal the potential for a 'liquid gold' biopsy.Br J Cancer. 2024 Mar;130(5):836-851. doi: 10.1038/s41416-023-02548-9. Epub 2024 Jan 11. Br J Cancer. 2024. PMID: 38212481 Free PMC article.
-
A prognostic six-gene expression risk-score derived from proteomic profiling of the metastatic colorectal cancer secretome.J Pathol Clin Res. 2022 Nov;8(6):495-508. doi: 10.1002/cjp2.294. Epub 2022 Sep 22. J Pathol Clin Res. 2022. PMID: 36134447 Free PMC article.
-
Investigating Glioblastoma Multiforme Sub-Proteomes: A Computational Study of CUSA Fluid Proteomic Data.Int J Mol Sci. 2022 Feb 12;23(4):2058. doi: 10.3390/ijms23042058. Int J Mol Sci. 2022. PMID: 35216175 Free PMC article.
-
The Role of Network Science in Glioblastoma.Cancers (Basel). 2021 Mar 2;13(5):1045. doi: 10.3390/cancers13051045. Cancers (Basel). 2021. PMID: 33801334 Free PMC article. Review.
References
-
- Fu M.H., Wang C.Y., Hsieh Y.T., Fang K.M., Tzeng S.F. Functional Role of Matrix gla Protein in Glioma Cell Migration. Mol Neurobiol. 2018;55(6):4624–4636. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

